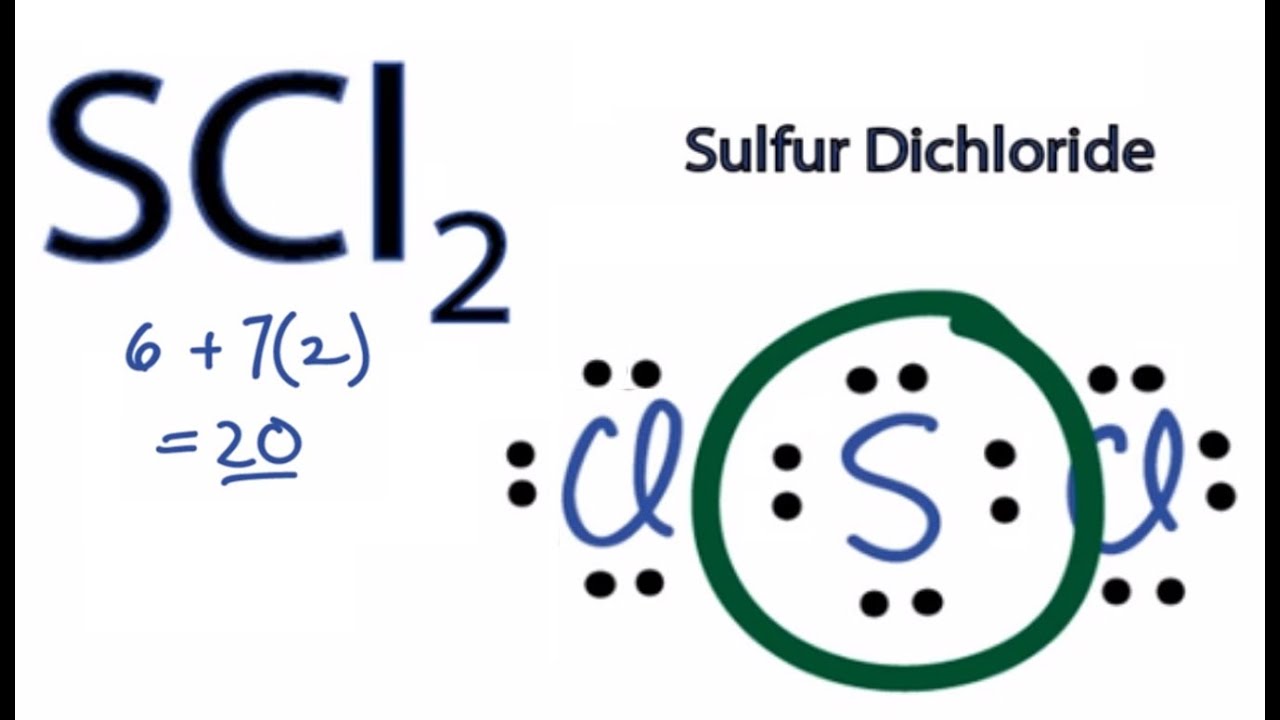
Scl2 Lewis Dot Structure
The formal charge on the bromine atom of SBr2 molecule V. Excess electrons that form lone pairs are represented as pairs of dots and are.
Draw The Correct Lewis Dot Structure For Nacl Ppt Video Online Download
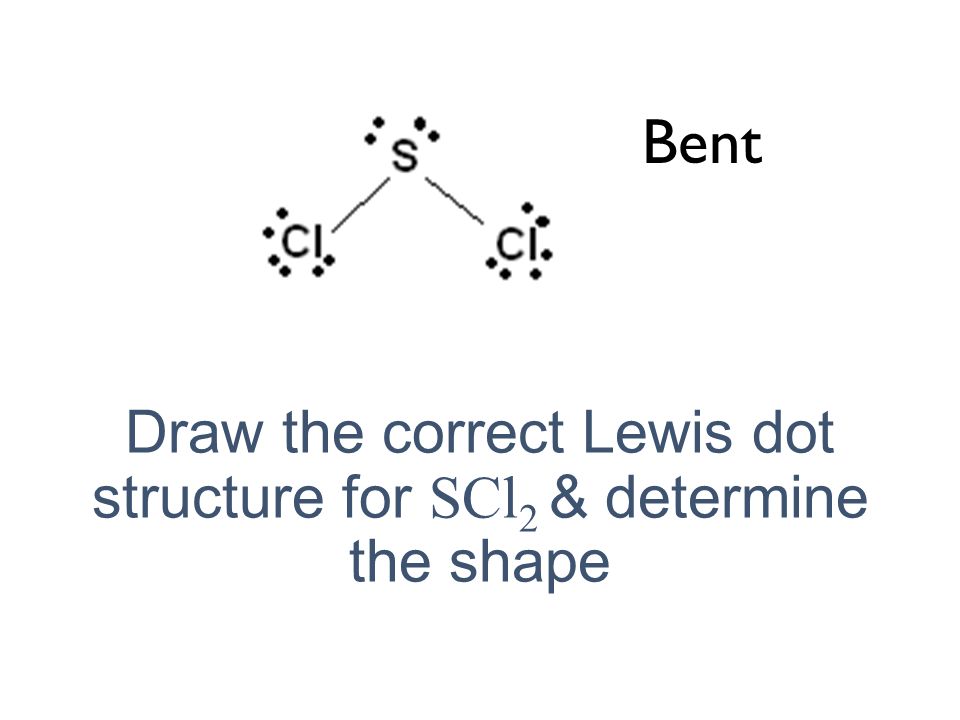
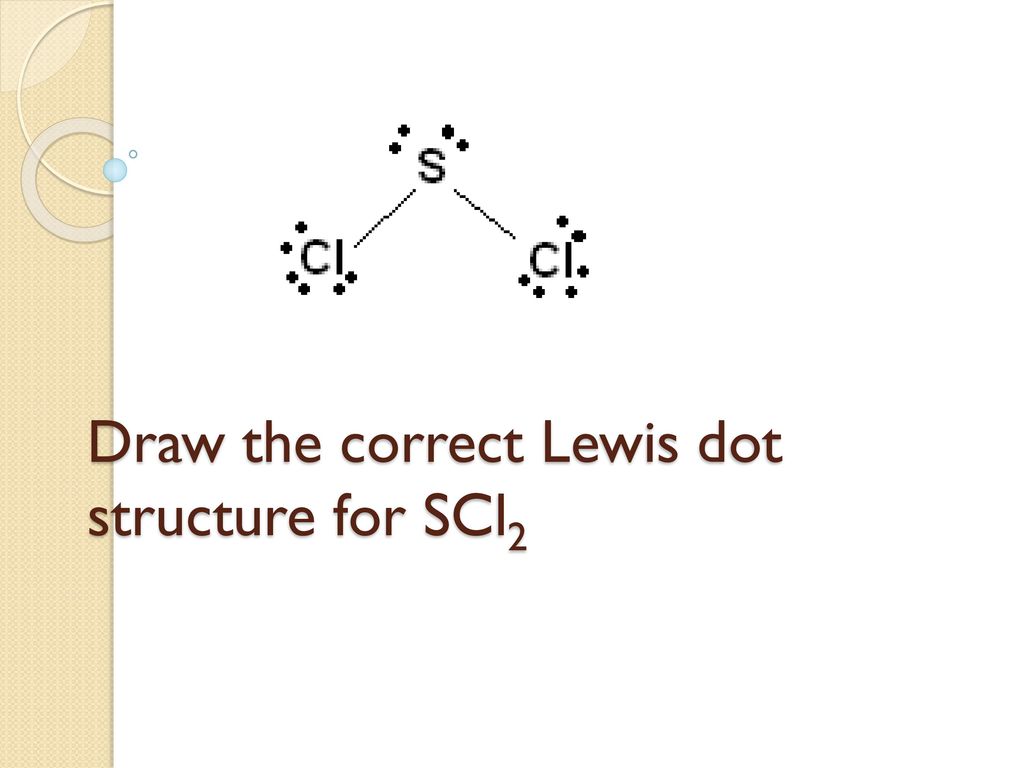
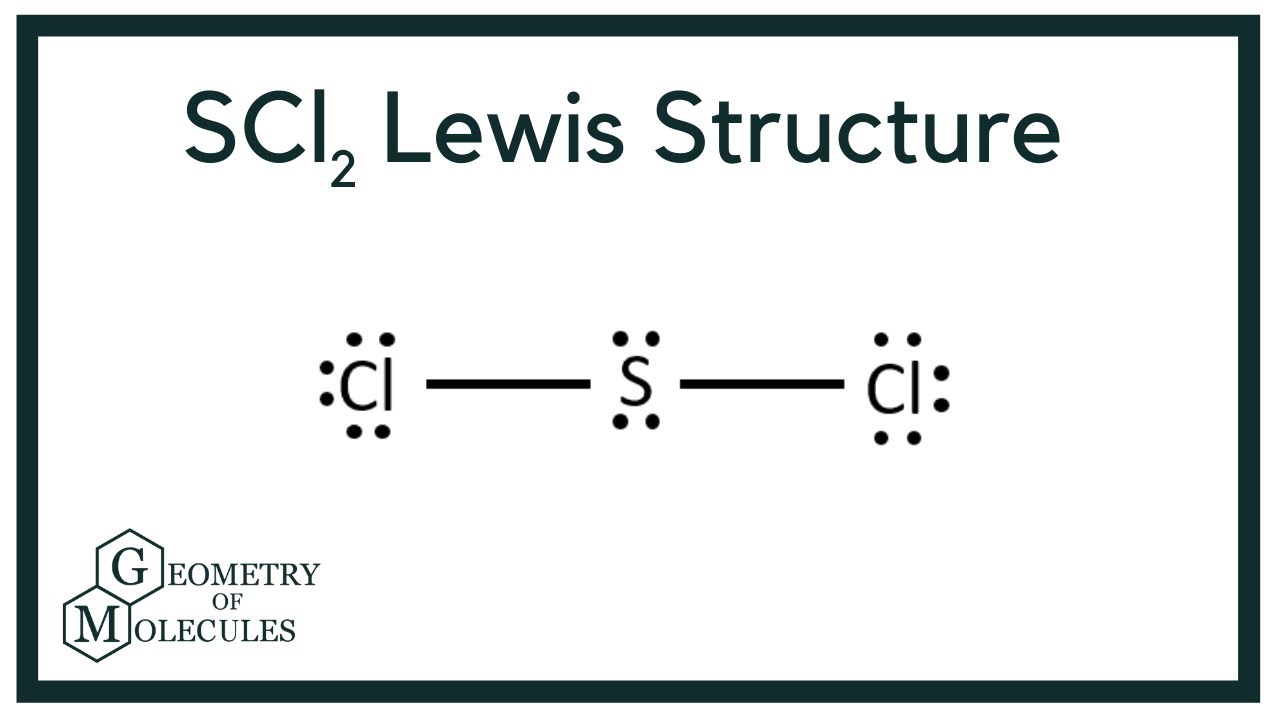
The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair.
Scl2 lewis dot structure. Chlorine group 7 or 17 has 7. The hybridization of each chlorine atom in Cl2 is Sp³. None of these require pi-bonding which is the method of formation for double and triple bonds.
Lets do the SCl2 Lewis structure. SCl2 lewis structure contains one sulfur and two chlorine atom. The electron dot structure of the CH3I molecule is also known as the CH3I Lewis structure.
Lets put the Sulfur at the center. Steps to draw electron dot structure or lewis structure of SCl2 Step 1. But we have two Chlorines so lets multiply that by 2.
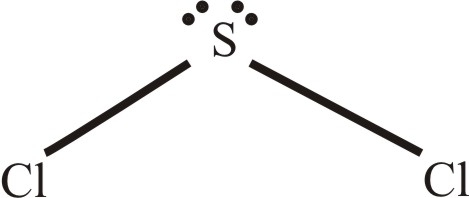
The outermost valence electrons of the CH3I molecule must be understood while considering the Lewis structure of the molecule. SBr2 Lewis dot structure. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms.
Its the least electronegative. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70.
It determines the number of outermost valence electrons as well as the electrons engaged in the CH3I molecules bond formation. Thus the hybridization of SCl2 is sp3. None of these require pi-bonding which is the method of formation for double and triple bonds.
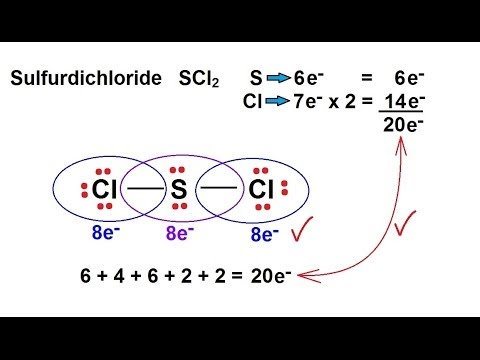
Six plus 14 equals 20 valence electrons. EBr LEBr 12BE VE Br Valence electron in a bromine atom of SBr2molecule. Sulfur being the less electronegative atom than chlorine placed at the center in lewiss diagram and chlorine spaced evenly around it.
A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride. Has a similar shape to water due to 2 lone valence pairs on the sulfur. 70 More Lewis Dot Structures This cherry-red liquid is the simplest sulfur chloride and one of the most common.
The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. It wont allow me to upload images. The molecular shape of Cl2 is linear.
Start with the molecules Lewis structure. The total valence electron available for drawing the Cl2 lewis structure is 14. To calculate the formal charge on the terminal bromine atom of the SBr2 molecule by using the following formula.
It might appear from the two dimensional drawing that the dipoles of the two S-Cl bonds should cancel one another to yield a nonpolar molecule. Sulfur and oxygen are in group 16 and bond similarly. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol.
Thus the hybridization of SCl2 is sp3. Cl2 is non-polar in nature because of no dipole moment present in it. I cant draw it but each atom has 6 electrons around it.
For the SCl2 Lewis structure use the periodic table to find the t. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. Jan 16 2015 SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm.
First you should count the total number of valence electrons in SCl2. Use your molecular models to explain why this molecule is polar. The formal charge of Chlorine in the Cl2 lewis dot structure is zero.
The Lewis Structure for SCl2 is given. The product contains about 72 - 80 SCl2 residual S2Cl2 and Cl2. Van Nostrand Reinhold Co 1987 p.
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center. On the periodic table Sulfurgroup 6 or 16has 6 valence electrons.
Stabilized pure sulfur dichloride should contain minimum 98 SCl2. For the scl2 lewis structure use the periodic table to find the total number of valence electrons for the scl2 molecule. Is SCl2 molecule polar or nonpolar.
Draw the Lewis electron dot structure for SCl2 and discuss its molecular geometry.
Bond Angle Of Scl2 Lewis Structures
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Chem 110 Exam 2 Flashcards Quizlet

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity
Valence Shell Electron Pair Repulsion

11 Draw Lewis Dot Structure For Scl2 And Determine Chegg Com

Does Scl2 Have A Dipole Moment Clutch Prep
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Types Of Bonding And Lewis Structures Ppt Download

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com
Solved Predict The Geometry Of Sulfur Dichloride Scl2 And The H Chegg Com
Scl2 Lewis Structure Sulfur Dichloride Youtube

How To Draw Lewis Structure For Scl2 Drawing Easy

The Lewis Diagram For Scl2 The Electron P Clutch Prep

What Is The Lewis Structure For Scl2 Study Com

What Is The Name Of The Hybrid Orbitals Us Clutch Prep