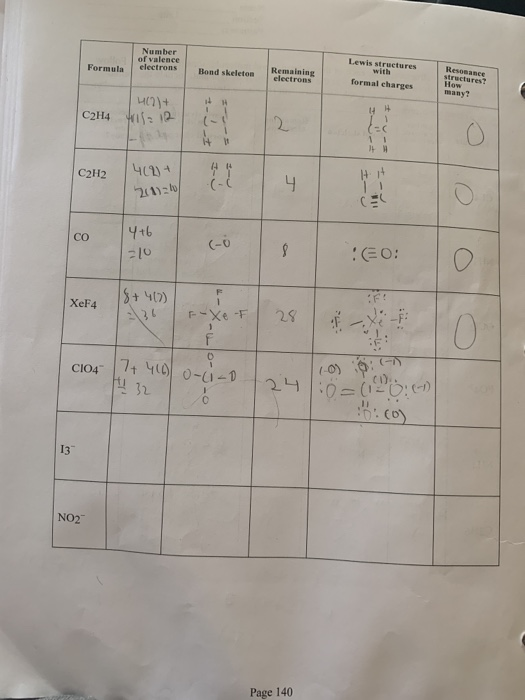
C2h4 Valence Electrons
To draw the Lewis structure for C2H4 the total number of valence electrons must be known. C2H4 Valence Electrons.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What is the total valence of C2H4.
C2h4 valence electrons. Total valence electrons given by hydrogen atoms 1 4 8. What is the electron dot formula of C2H4. So there are two valence electrons for Hydrogen atoms.
Hydrogen has an electronic configuration of 1s 1. Look at the structure in the 3rd step all hydrogen already got what they want. See the answer See the answer See the answer done loading.
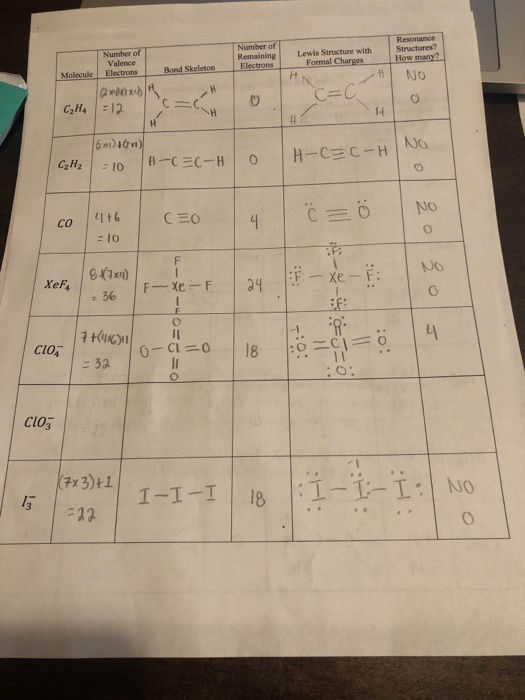
There are ten valence electrons in C 2 H. The molecular shape is predicted to be trigonal planar around each carbon atom. Therefore no addition or reduction of valence electrons due to charges.
Place remaining valence electrons starting from outer atom first. We know a square has four sidesAt firstwe have to place one valence electrons as dot to every side of that square before pairing up. Lets take a look.
Substance Lewis Structure Valence Electrons Zones e-Pair Molecular Geometry Shape C2H4 PC13 C2H6 C2H2 NH4 Xe0F4 C2H2Cl2 SO2 SOCl2 COCI2 This problem has been solved. For C 2 H 4 you have a total of 12 total valence electrons. Because total valence electrons in C2H4 are 12.
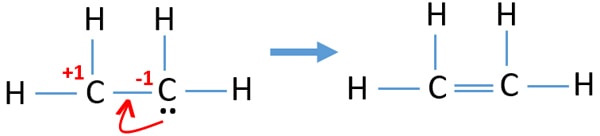
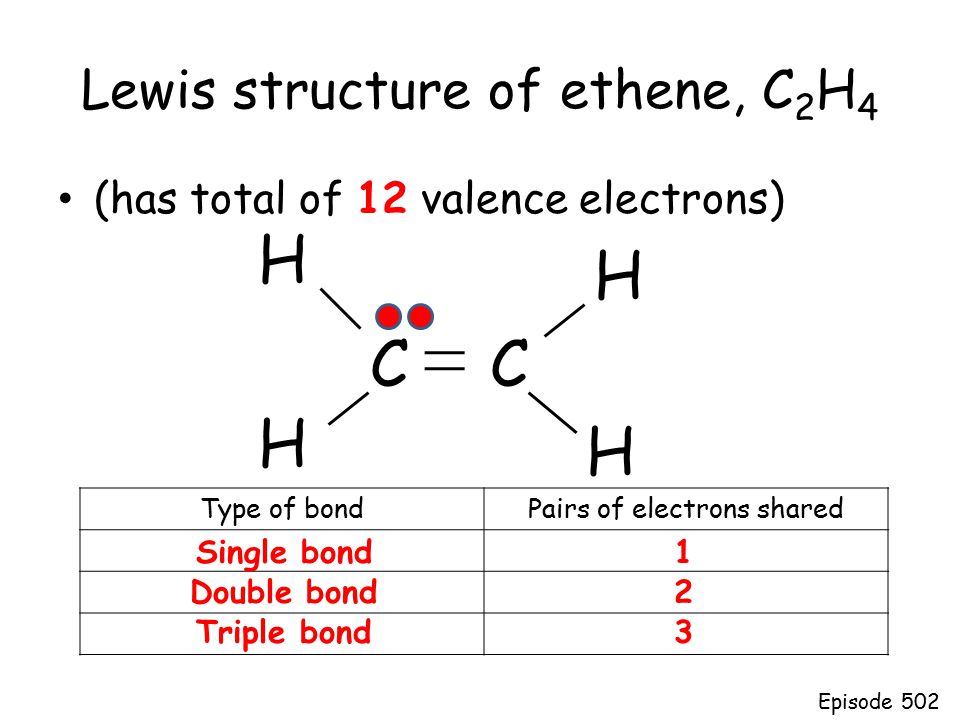
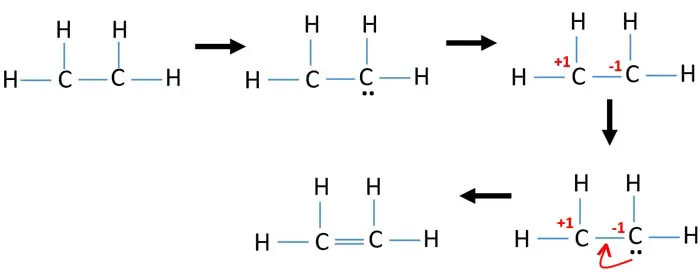
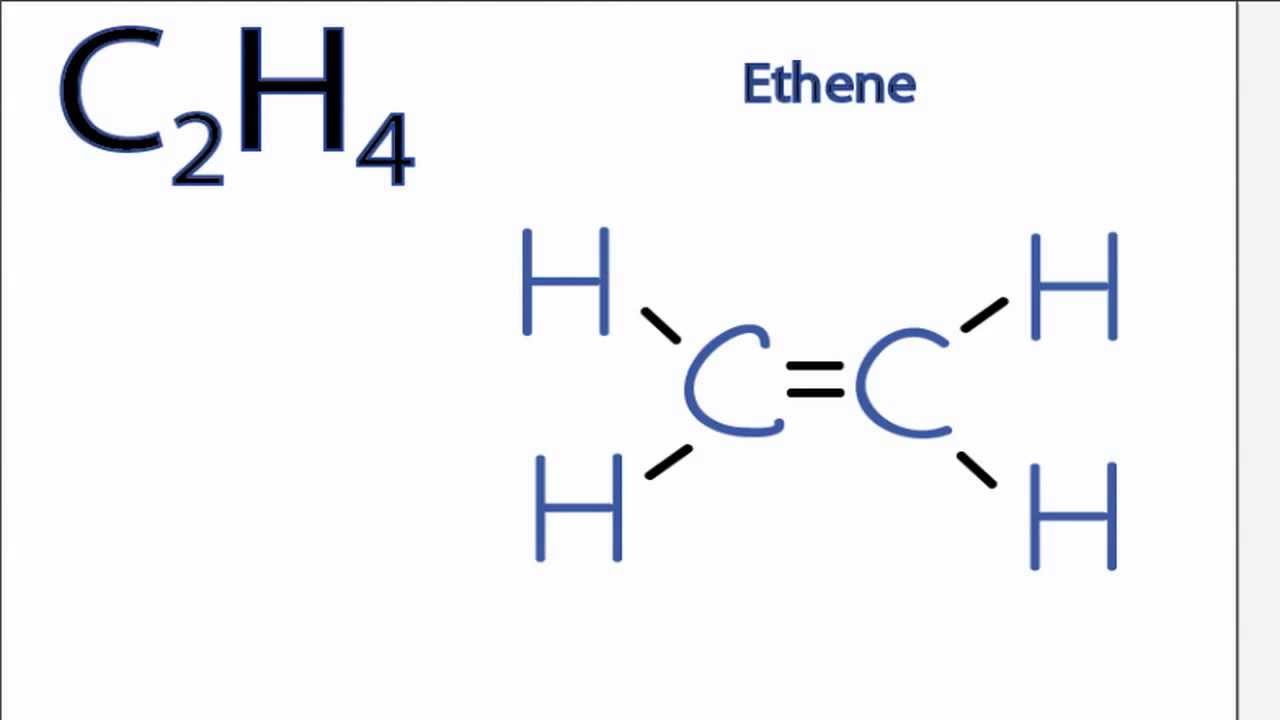
Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. This is composed of a σ framework and a π-bond. Total valence electrons given by two carbon atoms 4 2 8.
Hence there are eight valence electrons for Carbon atoms. Now we have to count total number of C2H4 Valence electrons. Again we have to find out the valence electrons of hydrogenH.
Also question is how many valence electrons are in c2o42. A Hydrogen atom has one valence electron in its outer shell. Total valence electrons 8 4 12.
So after using 10 electrons we are left with only 2 valence electrons. Its C2H4 and we want to write the dot structures for ethene. The carbon atom has four valence electrons in its outer shell but here as there are two Carbon atoms we will multiply the number by 2.
If we come way over here to Hydrogen its in group 1. Therefore the four Hydrogen atoms contribute 1 x 4 4 valence electrons. We will multiply it by two as there are two Hydrogen atoms.
As hydrogen only needs 2 electrons to complete its octet shell. It has 1 valence electron. It has a role as a human metabolite and a plant metabolite.
Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. Ethylene comprises two Carbon atoms with four Hydrogen atoms surrounding it. Carbon and Carbon are double bonded to each other with each carbon having two hydrogens attached.
Is C2H4 a geometry. There are four hydrogen atoms in ethene molecule Therefore. Number of valence electrons 18Organometallics large ie.
Strong field organometallic ligands like CO C2H4 C5H5 π-acceptorswhich stabilise t2g orbitals- see Coordination Chemistry Handout page 27Hence t2g now bonding fully occupied 6e and eg antibonding unoccupied. Oxalate2- is a dicarboxylic acid dianion obtained by deprotonation of both carboxy groups of oxalic acid. Therefore the two Carbon atoms contribute 4 x 2 8 valence electrons.
Electron dot structure of C2H4 H2CCH2. Valence electrons in C 2 H 2. The nunber of valence electrons in carbon is 4.
Carbon is in group 4 of the periodic table with the electronic configuration He 2s 2 2p 2. 2C 4 2 8 4H41 4 Sothe total number of C2H4 valence electrons is twelve 8 412These 12 valence electrons have two tasks at the the same timeThey connect all the atoms and satisfy the duet rule of hygrogens and octet rule of two carbon atoms. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Also Know what c2o4 2. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. All electrons are shared within bonds in this compound.
8 2 10. There are no charges in ethene molecule. Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure.
To do that we always count our valence electrons up first. It is an oxalate and a dicarboxylic acid dianion. Carbon has 4 and hydrogen has 1 making a grand total of 12 e- 42 41.
Ethylene C2H4 has the Lewis Structure. 34 36 and 38 valence electrons were studied. Nowto draw the lewis dot structure of carbonwe need to imagine a square around carbon.

C2h4 Molecular Geometry Shape And Bond Angles Youtube

Ethene C2h4 Lewis Structure Hybridization

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts
Number Of Valence Electrons Number Of Remaining Chegg Com

Covalent Bonding Write Down The Information On These Slides So That We Can Move Through Them Quickly Tomorrow Ppt Download
Let S Review Oxygen O 8 1s2 2s2 2p4 Oxygen Has 6 Valence Electrons Ppt Download

Draw The Lewis Structure For The C2h4 Ske Clutch Prep
Ethene C2h4 Lewis Structure Hybridization

Drawing The Lewis Structure Of C2h4 Youtube
Lewis Structures Number Of Valence Electrons Ressance Chegg Com
Lewis Electron Dot Structures Ck 12 Foundation

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
Is C2h4 Polar Or Nonpolar Youtube
Lewis Structure Of C2h4 Biochemhelp
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry


