Clof2 Lewis Structure
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu. Sketch the Lewis structures of ClF2 and ClF2.
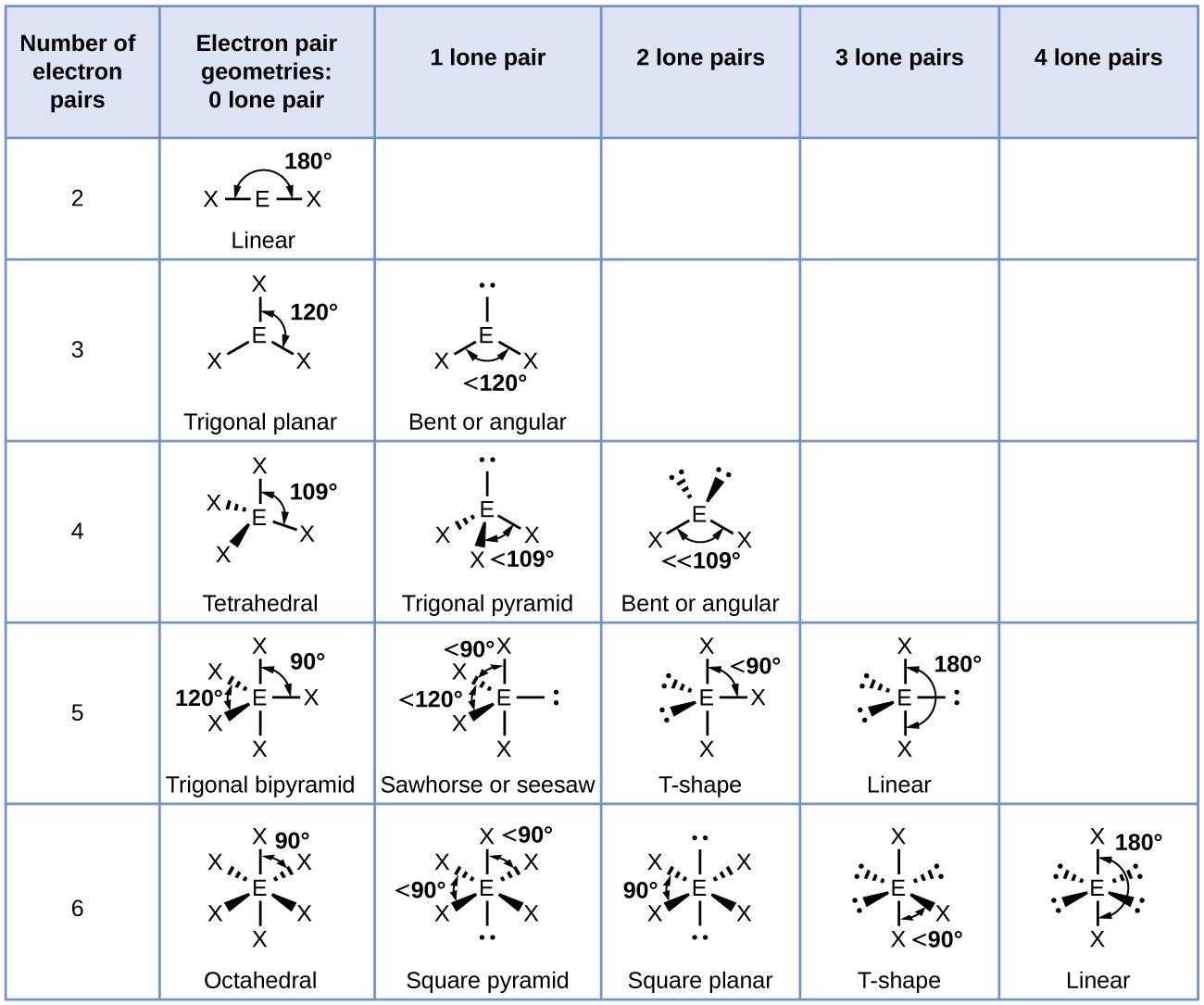
7 6 Molecular Structure And Polarity Chemistry
Rather it is a resonance hybrid of them both Lewis Dot Structure of ClO2- Chlorite Ion.

Clof2 lewis structure. There are total of 20 valence electrons for the ClO2- Lewis structure. What are the electron-pair and molecular geometries of each ion. I also go over hybridization shape and bond angles.
A quick explanation of the molecular geometry of ClO2 - Chlorite ion including a description of the ClO2 - bond anglesLooking at the ClO2- Lewis structure. CO2 Lewis Structure. In the Lewis structure for COF 2 there are a total of 24 valence electrons.
Out of 7 valence electrons on chlorine 2 electron pairs involve in formation of 2. Solutions for Chapter 10 Problem 24GQ. Based on nonempirical calculations by the Hartree-Fock-Roothaan method an explanation for the dependence of the structure of the ClOF2 cation on the nature of the anion was proposed.
The actual structure is nine of these. What hybrid orbital set is used by Cl in each ion. Lewis diagram is a representation of the valence electron within a molecule.
OCl is the hypochlorite ion the active ingredient in chlorine laundry bleach and swimming pool disinfectant. To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets. A step-by-step explanation of how to draw the ClO- Lewis Dot Structure HypochloriteFor the ClO- structure use the periodic table to find the total number.
See the Big List of Lewis Structures. Chloryl fluoride ClO2F or ClFO2 CID 139523 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. To draw the lewis diagram of any molecule we have to follow 5 or 6 simple steps depending on the complexity of the molecule.
Youll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon. Remember that the negative sign counts as one valence electron. The Lewis electron structure is drawn within brackets as is customary for an ion with the overall charge indicated outside the brackets and the bonding pair of electrons is indicated by a solid line.
For the Lewis structure for ClO2 you should take formal charges into account to find the best Lewis structure for the molecule. Remember that the negative sign counts as one valence electron. I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide.
Drawing the Lewis Structure for ClO 2 The ClO2 Lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. In the ClOF2 HF2 and ClOF2 BF4 HF systems this cation exists as a pyramidal structure C s symmetry while in the ClOF2 AuF6 HF system it exists as a planar structure C 2v symmetry. Do both have the same F Cl F angle.
Chlorine dioxide molecule ClO2 has central Chlorine atom attached to 2 Oxygen atoms via two double bonds. The lewis structure of CO2 can be with some simple steps but before that it is important to understand lewis structure properly. Two lone pairs present on the central atom of the ClO2- Lewis structure.
Answer to Draw the lewis structure and sketch the 3D structure of ClOF2 and ICl4-. According to the octet rule an atom attains stability by fulfilling its octet. In the ClOF2 HF2 and ClOF2 BF4 HF systems this cation exists as a pyramidal structure C s symmetry while in the ClOF2 AuF6 HF system it exists as a planar structure C 2v symmetry.
So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. Based on nonempirical calculations by the Hartree-Fock-Roothaan method an explanation for the dependence of the structure of the ClOF2 cation on the nature of the anion was proposed. This gives us two new structures in which one O atom has FC -1 and the other atoms have FC 0.
Lewis structure of ClO2- contains one single bond and one double bond. For example in CO2 carbon needs 6 electrons to.
7 6 Molecular Structure And Polarity Chemistry

Identify The Hybridization Of The Central Atom In Each Of Th Clutch Prep

Lewis Acidic Behavior Of Moof4 Towards The Alkali Metal Fluorides In Anhydrous Hydrogen Fluoride Solutions Stene 2019 European Journal Of Inorganic Chemistry Wiley Online Library
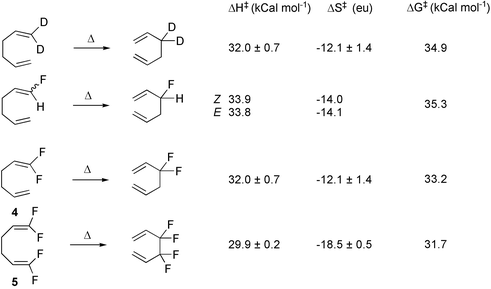
Syntheses Of Selectively Fluorinated Cyclodecenones The First Deployment Of The Neutral Oxy Cope Rearrangement In Organofluorine Chemistry Organic Biomolecular Chemistry Rsc Publishing Doi 10 1039 B311261f
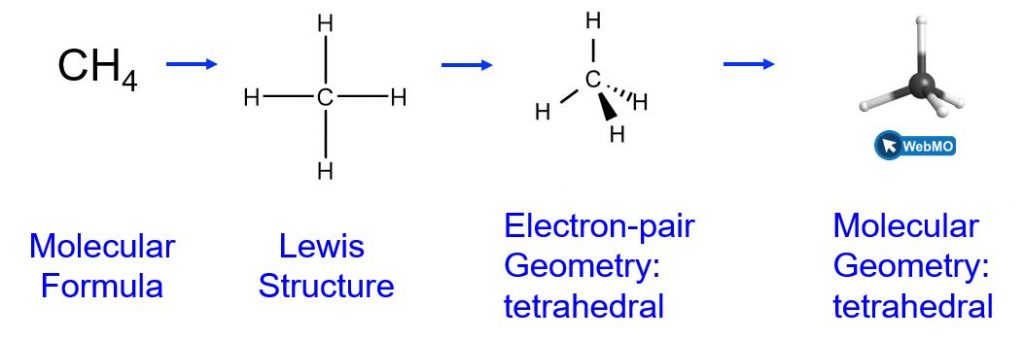
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book

Identify The Electron Pair Geometry And Th Clutch Prep

Lewis Acidic Behavior Of Moof4 Towards The Alkali Metal Fluorides In Anhydrous Hydrogen Fluoride Solutions Stene 2019 European Journal Of Inorganic Chemistry Wiley Online Library

Identify The Electron Pair Geometry And Th Clutch Prep

Identify The Hybridization Of The Central Atom In Each Of Th Clutch Prep

Lewis Acidic Behavior Of Moof4 Towards The Alkali Metal Fluorides In Anhydrous Hydrogen Fluoride Solutions Stene 2019 European Journal Of Inorganic Chemistry Wiley Online Library

7 6 Molecular Structure And Polarity Chemistry
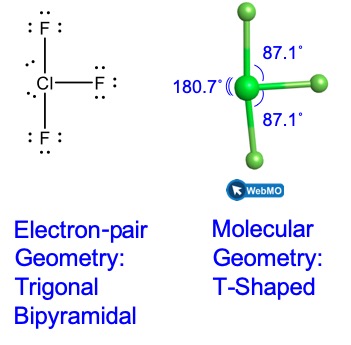
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
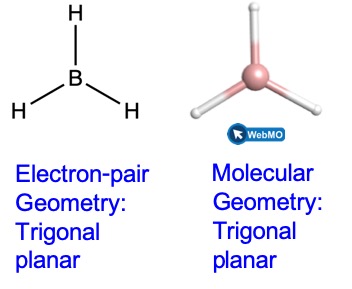
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
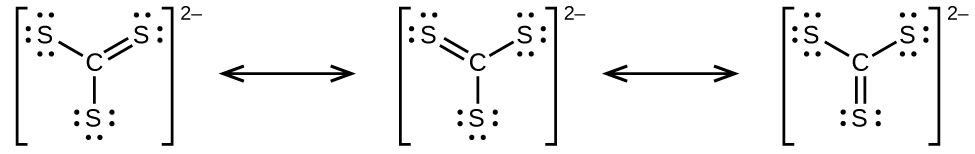
7 6 Molecular Structure And Polarity Chemistry

How To Draw The Lewis Structure For Clf2 Youtube

Lewis Acidic Behavior Of Moof4 Towards The Alkali Metal Fluorides In Anhydrous Hydrogen Fluoride Solutions Stene 2019 European Journal Of Inorganic Chemistry Wiley Online Library
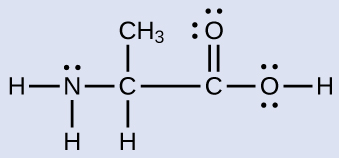
7 6 Molecular Structure And Polarity Chemistry

Identify The Electron Pair Geometry And Th Clutch Prep
