Xef2 Lewis Structure Lone Pairs
CH 3 Br Methyl Bromide Bromomethane Lewis Structure and Steps of Drawing. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible.

Xef2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table.
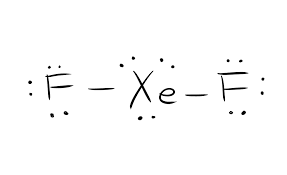
Xef2 lewis structure lone pairs. D What are the bond angles. This distortion increases with the number of lone pairs. Oxygen is the central atom in waterit has two lone pairs of electrons the bond angle is 10427 The ideal H-O-H bond angle ought to have been 10928.
E Is this a polar. What is the electronic geometry of this molecule look at atoms and lone pairs. Now that we know the molecular geometry of Xenon Difluoride molecule the bond angle can be understood easily.
Shape of XeF2 is Linear though Xe is sp3d hybridized to give trigonal bipyramidal structure where three lone pairs of electrons occupy three corners of equilateral triangle and two F atoms occupy axially giving it linear shape. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Lewis structures also called electron-dot structures or electron-dot diagrams are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
We will learn how to draw lewis structure of CH 3 Br step by step in this tutorial. Experts are tested by Chegg as specialists in their subject area. Draw the Lewis structure for XeF2 a How many groups atoms and lone pairs surround the central oxygen.
Both bond pair and lone pair of electrons are shown in this type of. The presence of lone pairs causes slight distortion in the bond angles of molecules. The VSEPR predicts the linear shape.
The lone pairs take up the most space so they occupy the equatorial positions. There are five electron regions Steric Number 5 about the central carbon atom. As lone pairs of electrons do contribute to shape XeF2 has linear shape as.
It has only one carbon atom. Remember that Xenon can have more than 8 valence electrons. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
Show all lone pairs. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. Elements in the first 2 periods of the Periodic Table do not have access to the d sublevel and must adhere to the octet or duet H and He rule.
There are a total of 22 valence electrons in the Lewis structure for XeF2. ABOUT XeF2- It is a very good fluorination agent. They are the three lone pairs and the two Xe-F bonds.
XeF 2 is dsp 3 hybridized and contains 3 lone pair and 2 bonding pairs of valence electrons around the Xenon. The VSEPR theory predicts that XeF₂ is linear. Lewis dot structure is a representation of a covalent molecule in which the distribution of electrons on atoms is shown as dots.
Lewis structure of CH 3 Br contains 3 C-H bonds and 1 C-Br bond. Draw this VSEPR structure next to the Lewis structure. This tells us that there are five electron regions Steric Number 5 about the central carbon atom.
Carbon atom is the center atom and bromine atom has 3 lone pairs. XeF2 is a linear molecule due to the arrangement of fluorine atoms and the lone pairs of electrons in the symmetric arrangement. What is the lewis structure of XeF2O.
C What is the molecular shape of this molecule. We review their content and use your feedback to keep the quality high. Who are the experts.
We must first draw the Lewis structure for XeF₂. Chemistry QA Library In the BEST Lewis structure for the molecule XeF2 how many non-bonding lone pairs 1 pair 2 of electrons are found on the central atom In the BEST Lewis structure for the molecule XeF2 how many non-bonding lone pairs 1 pair 2 of electrons are found on the central atom. A Lewis structure can be drawn for any covalently-bonded molecule as well as coordination compounds.
They are the three lone pairs and the two Xe-F bonds. Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. There are two pairs of bonded electrons and three lone pairs of electrons.

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube
Does The Vsepr Theory Predict That Xef2 Is Linear Socratic

Xef2 Molecular Geometry Bond Angles Electron Geometry Youtube

Xef2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Shape Of The Xef2 Molecule And The Total Number Of The Lone Pair Present On Xe In A Xef2 Molecule Quora

Xef2 Lewis Structure Polarity Hybridization And Shape
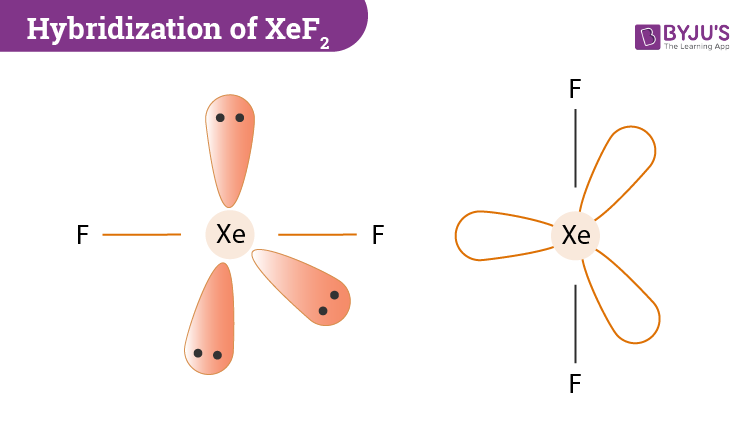
Hybridization Of Xef2 Hybridization Of Xe In Xenon Difluoride
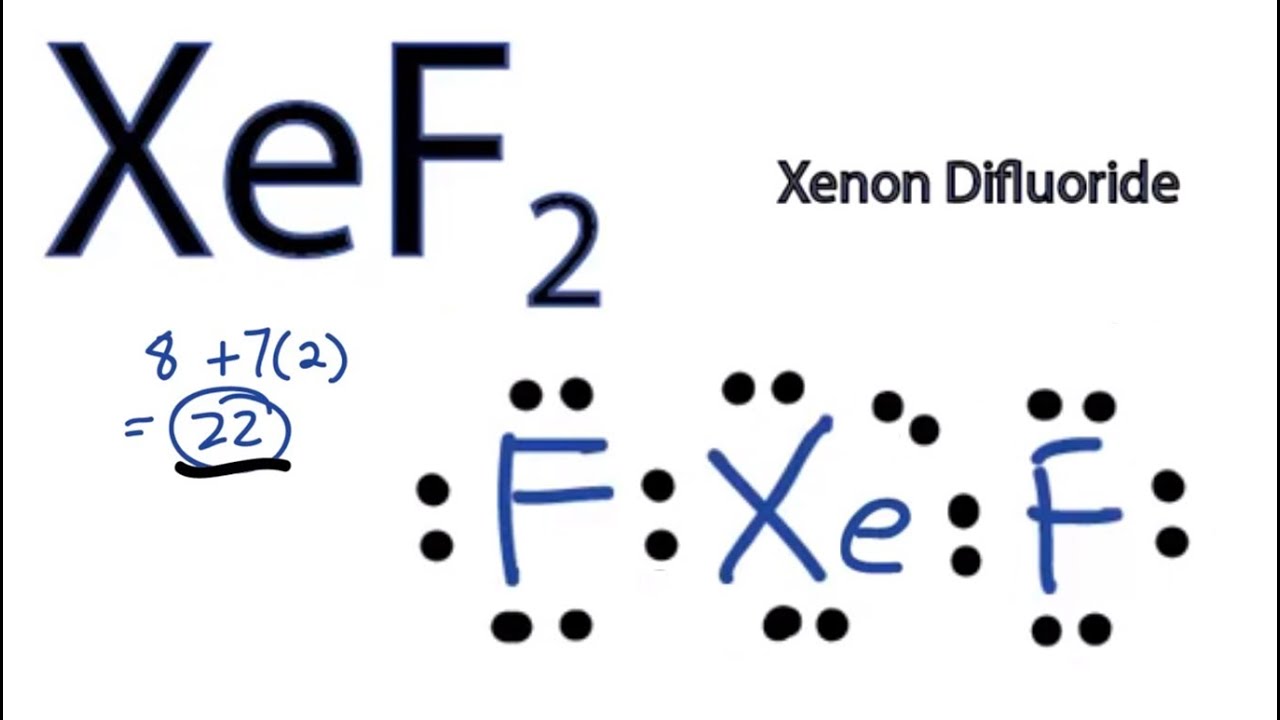
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube
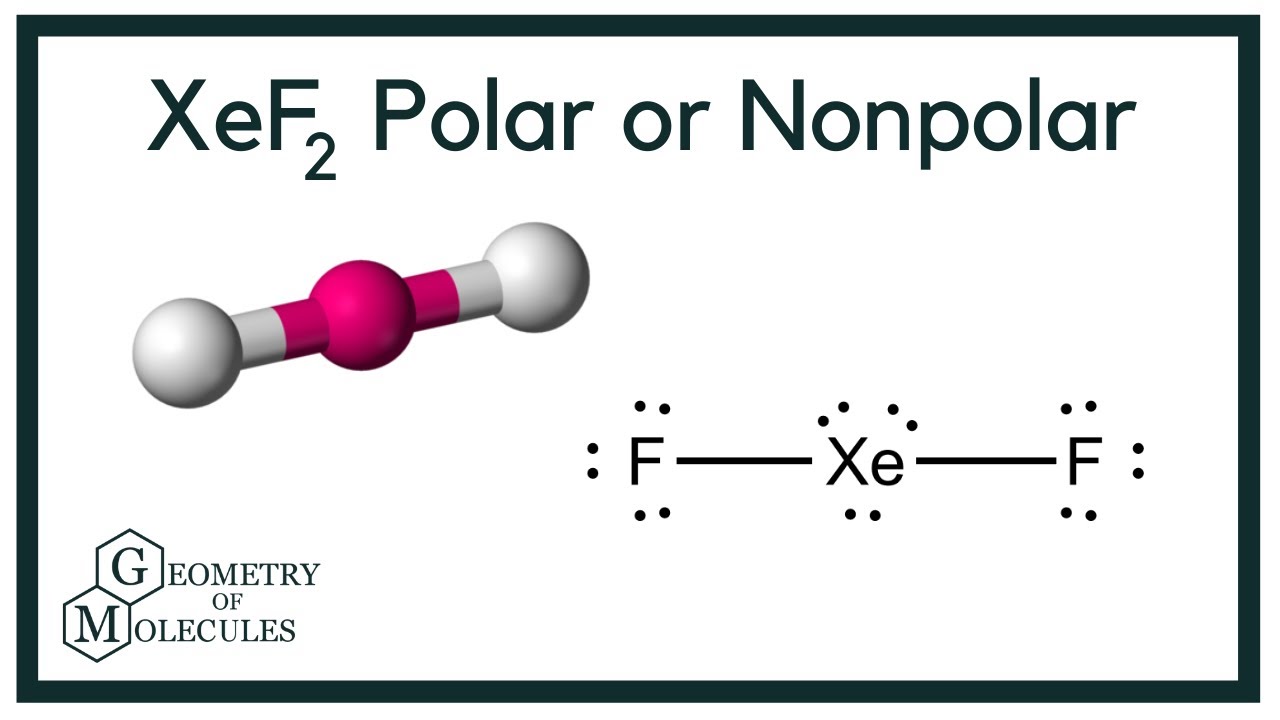
Xef2 Lewis Structure Polarity Hybridization And Shape
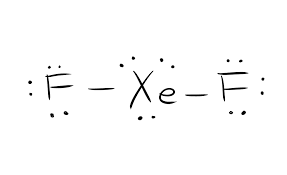
Xef2 Lewis Structure Polarity Hybridization And Shape

What Is The Molecular Shape Of Xef2 Quora

Number Of Lone Pairs And Bonding Pairs For Xef2 Xenon Difluoride Youtube
Does The Vsepr Theory Predict That Xef2 Is Linear Socratic

What Is The Shape Of The Xef2 Molecule And The Total Number Of The Lone Pair Present On Xe In A Xef2 Molecule Quora

What Is The Shape Of The Xef2 Molecule And The Total Number Of The Lone Pair Present On Xe In A Xef2 Molecule Quora
What Is The Molecular Shape Of Xef2 Quora

What Is The Shape Of The Xef2 Molecule And The Total Number Of The Lone Pair Present On Xe In A Xef2 Molecule Quora
Xef2 Lewis Structure Polarity Hybridization And Shape

Xef2 Lewis Structure Polarity Hybridization And Shape