H2co Lewis Structure Hybridization
First write down the expanded structure of the compound correctly with all bonds shown. This results in the hybridization with 1 s orbital and 2 p orbitals so sp2.
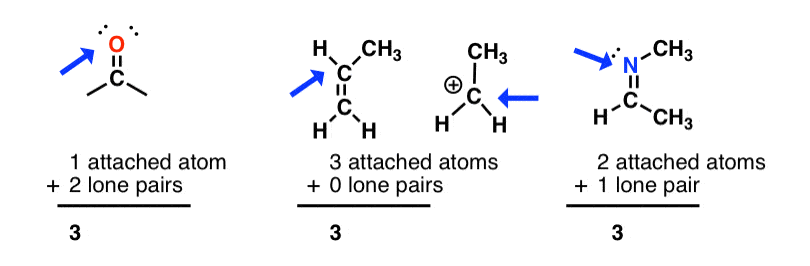
Lewis Structures And Hybridization Quiz Quizzma
-The valence electron configuration shows that C has a full 2s orbital and two 2p orbitals which are not full.

H2co lewis structure hybridization. Moreover if you realize the formal charge in the Lewis structure having a double bond is completely neutralized. As another example the molecule H2CO with Lewis structure shown below has 3 electron groups around the central atom. Calculate the total valence electrons in the molecule.
Two to Hs and a double bond to O. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond. For example given H2CO the C has an sp2 hybridization because it only goes it 3 directions.
π bonds between the same. Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. H 2Te a Te is in Group VI so Lewis structure is analogous to H 2O first structure b VSEPR 2 bp 2 lp 4 shape is tetrahedral c Molecular shape is bent d Hybridization is sp3 VSEPR 4 pairs on central atom so need 4 orbitals e Polar.
Lewis dot structure of H 2 CO Alternatively a dot method can be used to draw the lewis structure. B Determine the hybridization scheme bond angles and the pie and sigma bond present in the molecule. H 2 CO is the simpliest example of the organic functional group called the Aldehydes.
H1x22 C4 O Total b Draw the Lewis structure for H2CO. The repulsion between these groups produce a trigonal planar geometry with. What is the hybridization of oxygen in CO 2.
A Draw the correct Lewis structure. EN H-Te 0. Lewis dot structure of H 2 CO.
D Calculate the partial charges for each atom. If the carbon is connected to four other atoms its hybridisation is sp3 easy to remember. -Carbon has 3 electron groups therefore there are 3 sp2 orbitals with 1 electron in each.
Each oxygen has two lone pairs and forms one s bond and one p bond. Molecular Geometry of CH2O The CH2O is a tetra atomic molecule where the bond angles for the hydrogen-carbon-hydrogen H-C-H and hydrogen-carbon-oxygen H-C-O are 116 and 122 and the structure is bent shaped. Visualize this by drawing the Lewis Structure of H2CO.
Then count the number of atoms connected to the carbon of interest to you. H 2 CO is also called Formaldehyde. What is the approximate H-C-O bond angle in formaldehyde H2CO.
Explain the hybridization of H2CO and the bond angles -The lewis structure shows that C is bonded to 2 H atoms and double bonded to an O. By knowing the Lewis structure we can also predict the three-dimensional geometry of an. The hybridization will be sp2 because the s orbital can only form 1 bond and the 2 p orbitals must be combined with the s orbital to allow for 3 bonds to be made by the central atom.
Carbon C is the least electronegative atom and goes at the center of the H 2 CO Lewis structure. This occurs when there are only 3 directions for the atom. σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds.
Carbon goes in the centreMake sure carbon and oxygen get 8 electrons to fulfil octet rule. Number of atoms sum of the superscripts. A 90 B 1095 C 120.
There are a total of 12 valence electrons in the H 2 CO Lewis structure. A I B II C III D IV. C What is the overall shape of the molecule.
In which structure is the hybridization incorrect. Which is not an acceptable Lewis structure for the anion CH2NCO. Use information from step 4 and 5 to draw the lewis structure.
A I B II C III. LEWIS STRUCTURE- HYBRIDIZATION. 1 for s and 3 for p.
For the molecule formaldehyde H2CO. The repulsion between these groups produce a linear shape for the molecule with bond angle of 180. This is sp 2 hybridization.
Question 1 11 Draw the Lewis structure of H2CO and Identify the hybridization of carbon in that structure 4 12 Draw Lewis structures for the following molecules including all major resonance contributors indicate the formal charge on each of the atoms in the structure 8 a N2 b CH3CO c 03 Question 2 21 Draw the structure of the following molecule and indicate the relevant. Why does sp2 hybridization occur. Alternatively a dot method can be used to draw the lewis structure.
Explain how σ and π bonds are similar and how they are different.
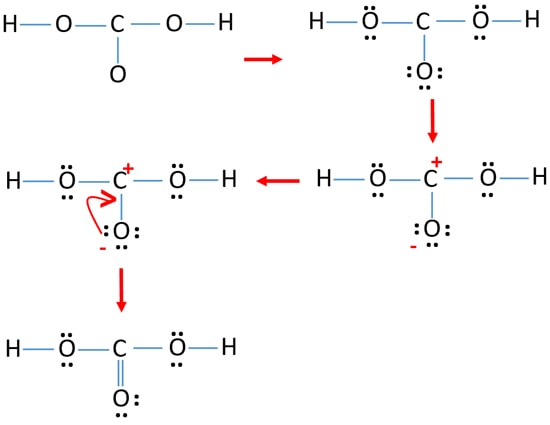
H2co3 Carbonic Acid Lewis Structure

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube

H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Co2 Lewis Structure Molecular Geometry And Hybridization

H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis
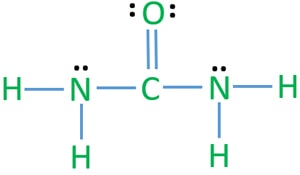
Urea Nh2conh2 Lewis Structure Steps Of Drawing
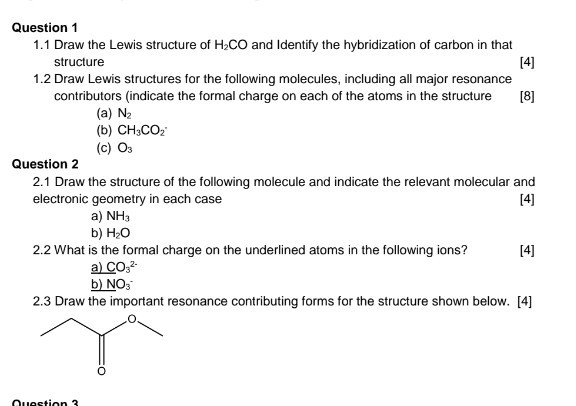
Question 1 1 1 Draw The Lewis Structure Of H2co And Chegg Com
Ch2o Lewis Structure Valence Electrons Hybridization Geometry Of Molecules

So3 Lewis Structure Sulfur Trioxide Youtube

Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube

H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula

H2co Lewis Structure How To Draw The Electron Dot Structure For H2co

Bonding In The Ch2o Molecule Youtube

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube

