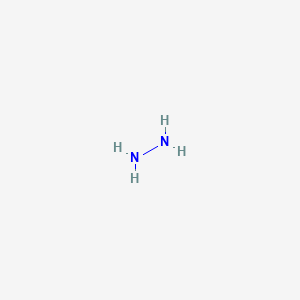
N2h4 Lewis Structure Molecular Geometry
Each nitrogen will form three bonds one with the other nitrogen and one. Become a member and unlock all Study Answers.
The molecular geometry of N2H4 is trigonal pyramidal and electron geometry is also tetrahedral.
N2h4 lewis structure molecular geometry. Hydrogen H only needs two valence electrons to have a full outer shell. Nitrogen has five valence electrons. Each Nitrogen atom forms a single bond with one Hydrogen atom and a double bond with the neighboring Nitrogen atom.
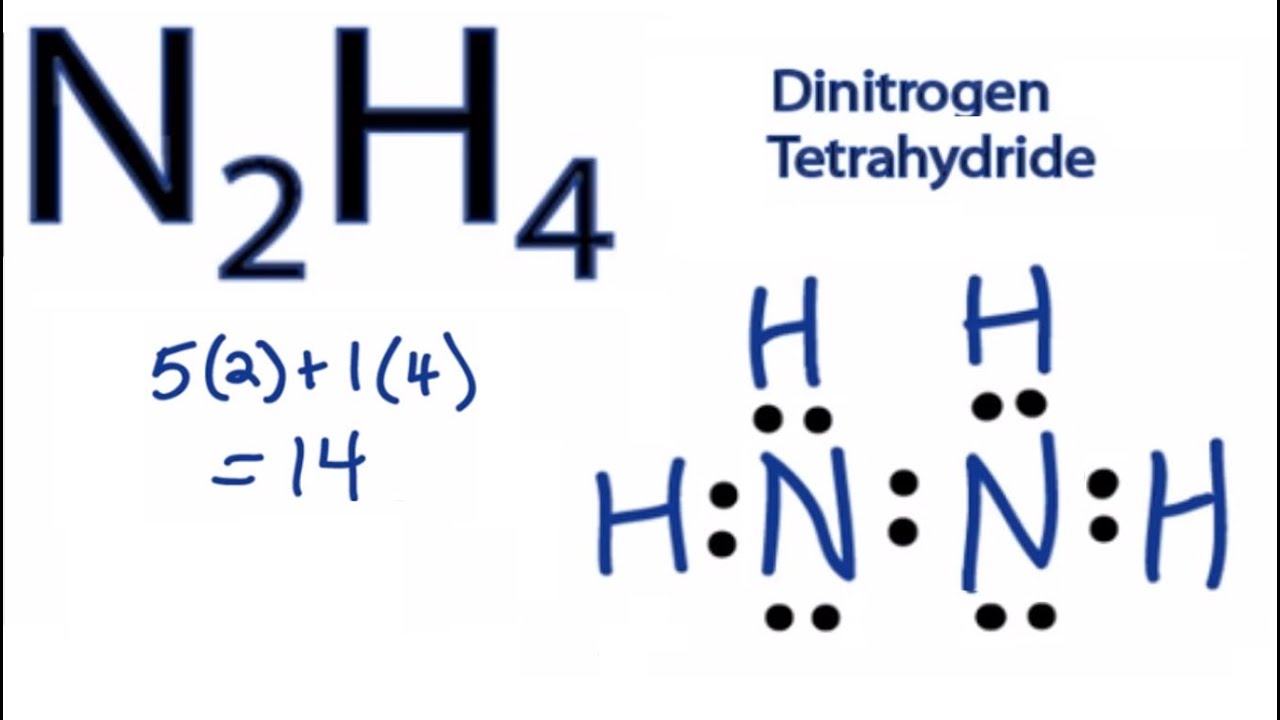
In the Lewis structure for N2H4 there are a total of 14 valence electrons. Experiment 12 Lewis Dot Structures and Molecular Geometry 12-2 Procedure for Determining Geometry Once the Lewis structure of a molecule or ion is determined the 3-D shape of the molecule can be determined. Show transcribed image text.
N2h4 Lewis Structure How To Draw The. Determine the molecular geometry of N2H4 skeletal structure The molecular geometry is about each interior nitrogen atom of n2h4 is Trigonal pyramidal. It can adapt two different geometrical forms cis and trans each with distinct symmetry.
This tutorial will help you deal with the lewis structure and molecular geometry for hydrazine n2h4. -please do molecular geometry for each. Lets do the N2H4 Lewis structure.
Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for hydrazine N2H4. Total 2 lone pairs and 5 bonded pairs present in N2H4 lewis dot structure. Lewis Structure of N2H2.
N2H4 Lewis structure. Lewis Structure is a 2D diagrammatic representation of a molecular or an ionic structure that helps us decipher the type of bond formation and gives us a simple overview of electronic arrangement. You have two Nitrogens.
Polar as the difference in electro negativities and bond arrangement creates a dipole moment. 1 Here is the Lewis structure. Hydrazine is a strong base and is highly unstable.
A positively charged polyatomic ion of Ammonium or NH4 comes into existence when an Ammonia atom goes through the process of protonation that is it loses one of its electrons and becomes positively charged. Determine the molecular geometry of N2H2 skeletal structure HNNH. There are three bond pairs and one lone pair around each.
Well determine the n2h4 molecular geometry with respect to nitrogen on right the other atom will have same shape since they are symmet. See full answer below. The molecule has a trigonal pyramidal shape with a bond angle less than 1095.
For total number v. It has four bonding pairs of electrons and two lone pairs of electrons. Let us find out the Lewis Structure of Dinitrogen dihydride N2H2.
Indicate the geometry about one central atom. - please do electron geometry for each. There are 12 valence electrons for this molecule.
To identify and have a complete description of the three-dimensional shape of a molecule we. NH4 Lewis Structure Molecular Geometry and Hybridization NH3 is the chemical formula of Ammonia. The first step towards sketching the Lewis Structure of any molecule is to understand the.
The lewis structure of the given molecule is. For structure calculate total number valence elect. Chemistry learning made easy.
-please draw the lewis structure for each. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Metallic The bonding in metals is characterized by delocalization of valence electrons.
Determine the electron geometry of N2H4 skeletal structure H2NNH2. Indicate the geometry about one central atom. This problem has been solved.
The Lewis structure of N2H4 N 2 H 4 is as follows. The Lewis structure model combined with valence shell electron pair repulsion VSEPR can be used to predict many structural features of covalently bonded molecules and ions. This problem has been solved.
The Valence Shell Electron Pair Repulsion theory or VSEPR theory is one useful theory for predicting the geometries of molecules. In the AlCl3 lewis structure a total of 9 lone pairs are present but no lone pair on the central atom. NO3 is a polyatomic ion with a negative charge.
The molecular geometry of AlCl3 is trigonal planar with each Al-Cl bond angeled 120 to each other and its electron geometry is also trigonal planar.

What Is The Molecular Shape Of N2h4 Quora

Hydrazine N2h4 And Carbon Disulfide Cs2 Clutch Prep

Draw The Lewis Structure For N2h4 Predict The Electron Geometry And Molecular Geometry And State Whether The Molecule Is Polar Or Nonpolar Study Com

Hydrazine N2h4 And Carbon Disulfide Cs2 Form A Chegg Com

Molecular Geometry Vsepr Ppt Video Online Download
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
N2h4 H2n Nh2 1 Lewis Structure 2 Perspective Chegg Com
Why Does N N Dimethylhydrazine Have Hydrazine When It Has N2h2 Not N2h4 Hydrazine Why Isn T It Called Dimethyldiimide Quora

What Is The Lewis Structure For N2h4 Study Com

Clo3 Lewis Structure Molecular Geometry Youtube
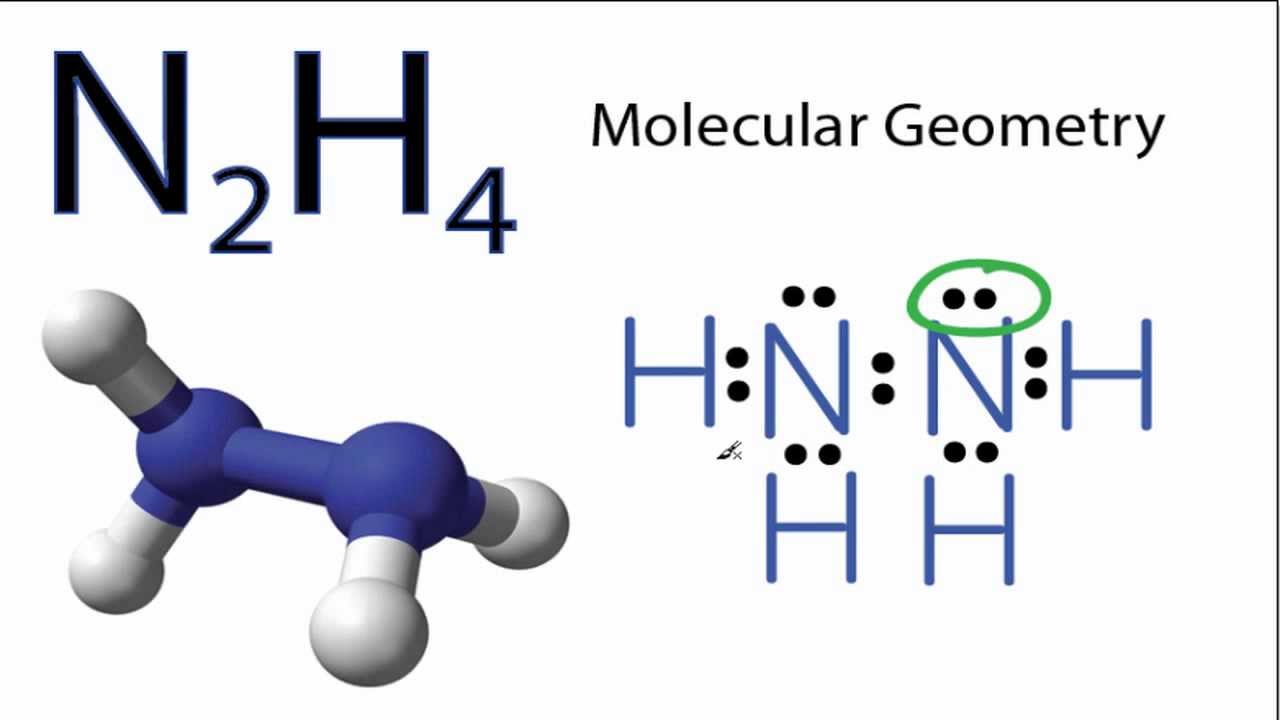
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
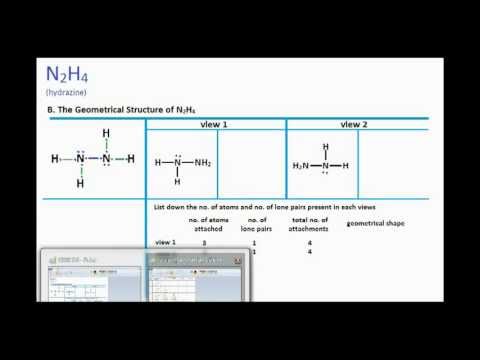
N2h4 Lewis Structure And Molecular Geometry Youtube
Ch4 Molecular Geometry Shape And Bond Angles Youtube

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape