Brf5 Lewis Structure Polar Or Nonpolar
Next as we draw the 3-d structure of BrF5 using the VSEPR rule. Put least electronegative atom in centre3.

Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity
One-Stop Logistics Services and Supply Chain Management Services.
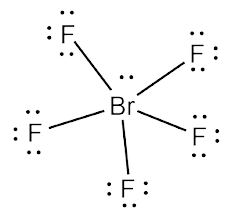
Brf5 lewis structure polar or nonpolar. The molecule has a central bromine atom surrounded by five fluorides and a pair of electrons. Brf5 polar or nonpolar. Drawing BrF5 Lewis Structure is very easy to by using the following method.
In this article we will study BrF5 lewis structure molecular geometry is it polar or non-polar. BrF5 or bromine pentafluoride is a polar molecule. BrF5 or Bromine Pentafluoride is a polar molecule as the molecular geometry of BrF5 falls out to be square pyramidal with an asymmetric charge distribution.
Bromine pentafluoride chemical formula is BrF5. The difference in electronegativity between the Bromine and Fluorine atoms also contributes to unequal charge distribution on the central Bromine atom. Brf5 lewis structure polar.
Here in this post we described. The total valence electron is available ICl2- lewis structure is 22. Phrases contain exact brf5 polar or nonpolar bond from credible sources.
The electron geometry of BrF5 in its Lewis structure is octahedral and the hybridization is sp3d2. Ill tell you the polar or nonpolar list below. The molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution.
The Lewis Structure Lewis Dot Diagram for IF51. A core bromine atom is surrounded by five fluorides and a pair of electrons in this molecule. So on this behalf BrF5 is Polar without a doubt.
The molecular mass of BrF5 is 174894 gmol. Quiz your students on Lewis Structure For BrF5 Molecular Geometry Bond Angle Hybridization Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. List molecules polar and non polar.
Is BrF5 Polar or Nonpolar Bromine Pentafluoride BrF5 is a polar molecule because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90. Put one electron pair in each bond4. As shown above due to its asymmetrical square pyramidal structure and the presence of a lone pair BrF 5 is considered a polar molecule.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. The total valence electron available for the BrF5 lewis structure is 42. The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral.
From the Lewis dot structure of BrF5 it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 296 and 398. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure Bromine pentafluorideFor the BrF5 structure use the periodic table to find the tota.
The polarity is best concluded by first drawing a Lewis dot structure for BrF5. Apr 10 2021 - BrF5 is interhalogen compound uses as a fluorinating. The polarity is best found by first drawing the Lewis dot structure for BrF5.
Aluminium chloride AlCl3 lewis structure molecular geometry polar or nonpolar hybridization bond angle Home Chemistry Article AlCl3 lewis structure and its molecular geometry Aluminium chloride is composed of aluminium and chloride also known as aluminium trichloride or aluminium III chloride having the chemical formula AlCl3. Lewis dot structure of BrF5. Therefore this proves that BrF 5 is a polar molecule.
Bromine pentafluoride is polar in nature. The difference between both the values is 102 which is greater than 04 so the BrF5 molecule is a polar. To better clarify your confusion look at the image below.
Related keywords of brf5 polar or nonpolar bond from credible sources. The hybridization of BrF5 is Sp³d². The steric number of iodine central atom in the ICl2- the molecule is 5 thus it forms Sp 3 d hybridization.
The nature of ICl2- is nonpolar because all dipole that generated along the bond will cancel out because of symmetrical geometry of it. Is BrF5 Polar or Nonpolar Because of its square pyramidal molecular structure asymmetric charge distribution and 90 bong angle bromine pentafluoride BrF5 is a polar molecule.
Is Brf5 Polar Or Nonpolar Bromine Pentafluoride Polarity Explained

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus
Determine Whether Each Molecule Is Polar O Clutch Prep

Is Brf5 Polar Or Nonpolar Molecular Geometry Of Brf5

Lewis Structure For Brf5 Molecular Geometry Bond Angle Hybridization Polar Or Nonpolar Quizalize

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas
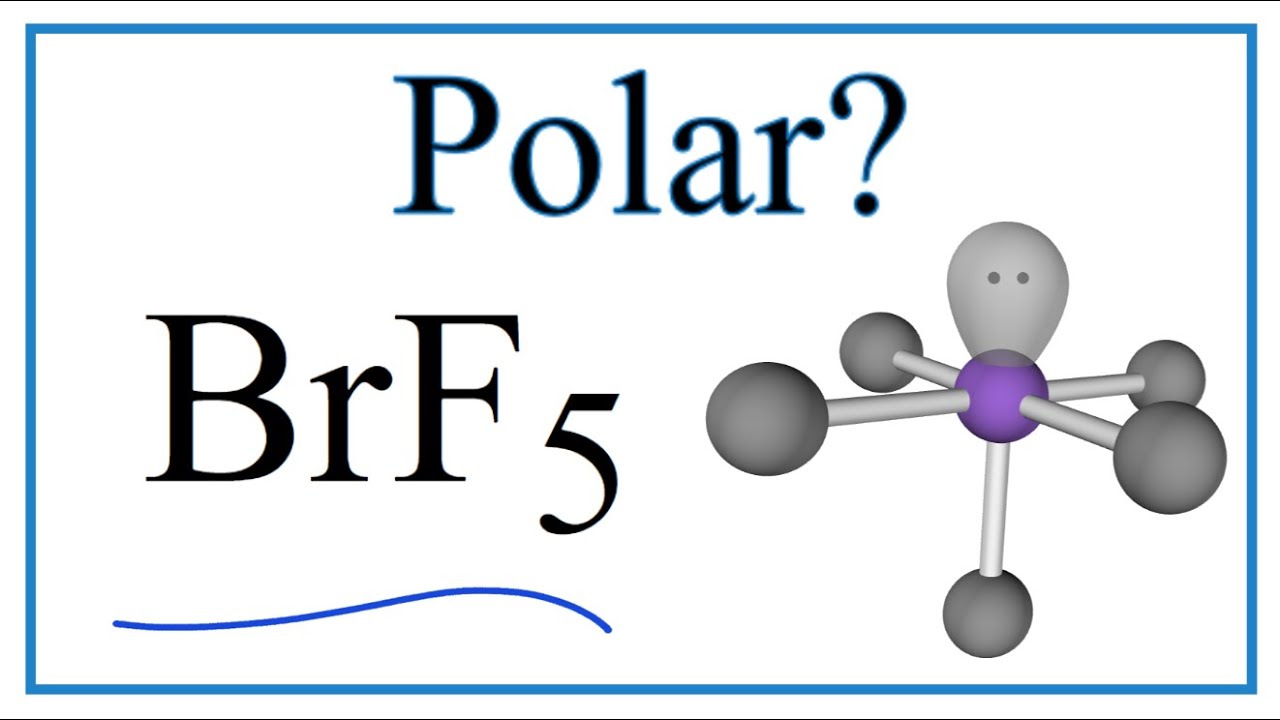
Is Brf5 Polar Or Nonpolar Bromine Pentafluroide Youtube

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Is Brf5 Polar Or Nonpolar Techiescientist

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Is Brf5 Polar Or Nonpolar Bromine Pentafluoride Polarity Explained

Is Brf5 Polar Or Nonpolar Techiescientist

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom
