C2h6 Lewis Structure Lone Pairs
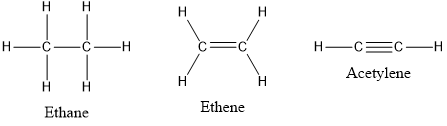
Every atom tends to complete its octet 8 electrons either by gaining or losing electrons except Hydrogen and Helium as they complete their duplet. No Comments on Lewis Structure of C2H6 Ethane Step-by-Step Ethane is two carbon atoms single-bonded together with three hydrogens on EACH of those two carbon atoms.
What Is The Molecular Geometry Of C2h6 Quora
Subtract step 3 number from step 1.
C2h6 lewis structure lone pairs. C2H6 is sp3 hybridised so form Tetrahedral geometry which has all H hydrogen out of plane hence non planar. Gives you bonding e-. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell.
The structures and geometries or the molecules can be different on the basis of the count of lone pairs. Each carbon in the Ethylene Lewis structure has Sp² hybridization and with two hydrogens it makes the structure look like a triangular planar which is two-dimensional. Lone pairs affect.
You must refer to the article specifically written on C2H6 Lewis Structure. Whereas C2H6 has Sp³ hybridization and its geometry is trigonal bipyramidal which is 3-dimensional. 12-12 0e-0 lone pairs.
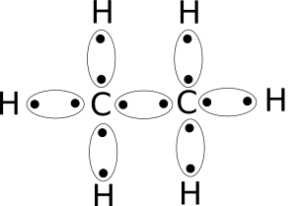
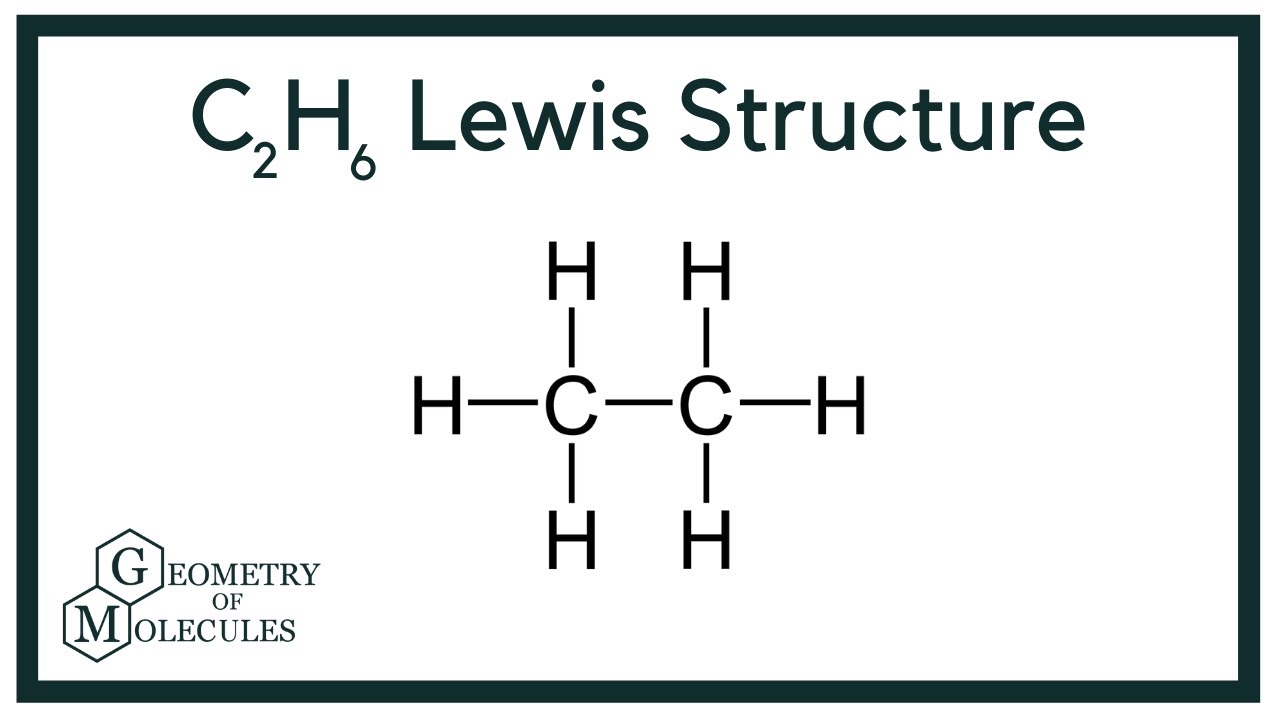
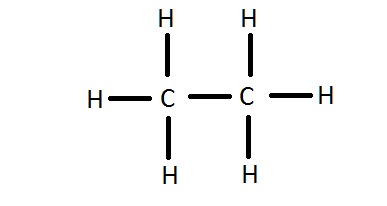
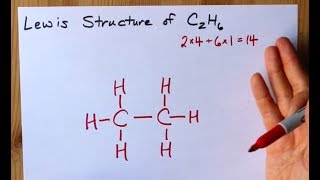
C2H6 Lewis Structure. Lewis structure is a 2D representation of the compound which represents only the valance shell electrons of the atoms in the molecule. 14e-2 7 bond pairs.
Find the number of nonbonding lone pairs e-. Hence Ethene is planar. There are no lone pairs or nonbonding pairs of electrons since all of the valence electrons are used up to form the molecules stable structure.
Subtract step 3 number from step 1. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells Total electron pairs are determined by dividing the number total valence electrons by two. Looking at the Lewis structure of c2h6 we can see that there are four atoms attached to the carbon of interest and there is no lone pair.
For C 2 H 2 total pairs of electrons are 5 102 in their valence shells. Lewis dot structure of C 2 H 6. Therefore there cannot be more than one stable resonance structure for C.
14-14 0 e-0 lone pairs. It is based on the octet rule ie. Carbon atoms bring 4 valence electrons each that makes 8 total valence electrons brought by carbon to this molecule.
Which Of The Following Molecules Has No Lone Pairs Of Electrons It Its Lewis Structure. Subtract step 1 total from step 2. Lewiss concept shows the bonding as well as the nonbonding electrons.
The first step in drawing the Lewis dot structure for ethane C2H6 is to determine how many valence electrons are available for the molecule. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells. C2h2 Lewis Structure Lone Pairs C2H2 Molecular Geometry Shape and Bond Angles YouTube Lewis dot structure for c2h2 ALQURUMRESORT C2H4 Lewis Dot Structure How to Draw the Lewis Structure Valence Shell Electron Pair Repulsion Theory.
Why C2H4 lewis structure is planar and C2H6 is non-planar. C2H6 C 2 H 6 will have smallest number of lone pairs on its Lewis structure. Find the number of nonbonding lone pairs e-.
A CH3CHO B CO2 C CH3Cl D C2H6 E None A CH3CHO B CO2 C CH3Cl D C2H6 E None This problem has been solved. In this molecule the electrons and atoms are symmetrically arranged. Find number of bonds by diving the number in step 3 by 2 because each bond is made of 2 e- 12e-2 6 bond pairs.
This means that the Lewis dot structure for C2H6 must account for 14 valence electrons either through bonding between atoms or through lone pairs. See full answer below. Alternatively a dot method can be used to draw the lewis structure.
In this compound only single bonds are present between. Calculate the total valence electrons in the molecule. Use information from step 4 and 5 to draw the lewis structure.

Solved Chapter 9 Problem 51e Solution Mastering Chemistry For Chemistry 11th Edition Chegg Com
What Is The Electron Dot Structure Of Ethane Quora

C2h6 Molecular Geometry Shape And Bond Angles Youtube
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape

C2h6 Ethanelewis Structure Clutch Prep
1 2 Lewis Theory Of Bonding Chemistry Libretexts
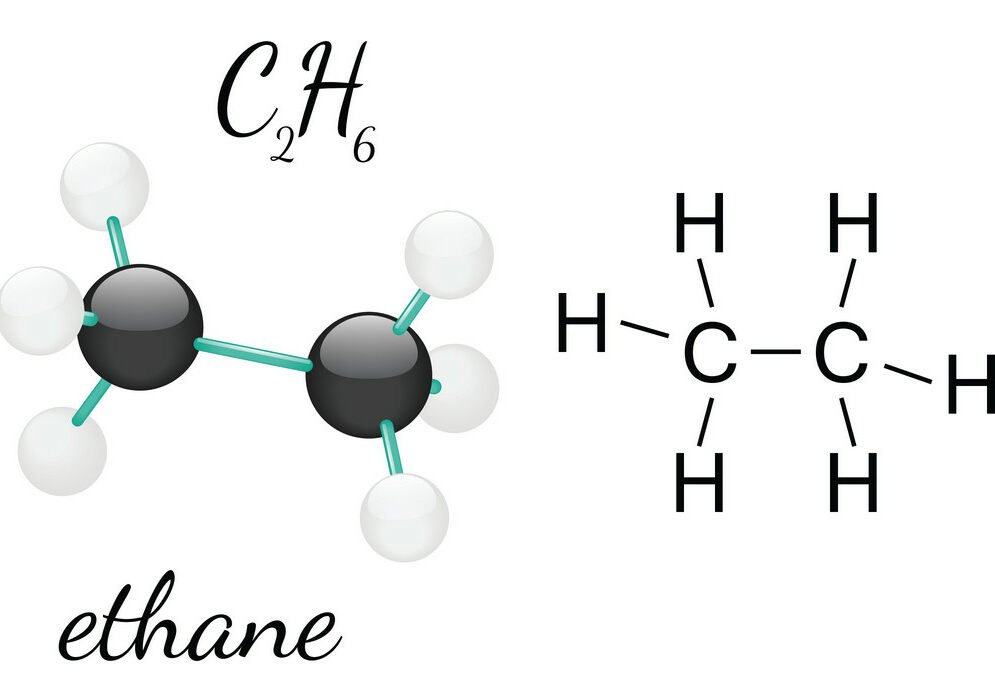
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
Observation 1 Geometries Of Molecules Chemistry Libretexts

Draw The Electronic Dot Structure Of Ethane Molecule C2h6
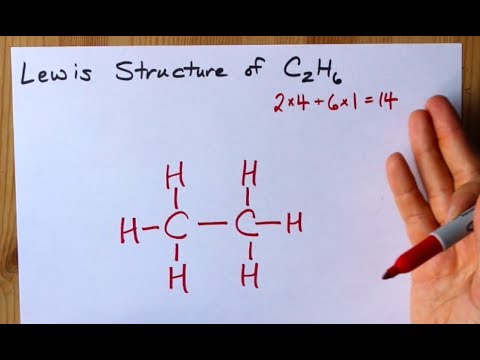
Lewis Structure Of C2h6 Ethane Youtube

C2h6 Ethanelewis Structure Clutch Prep
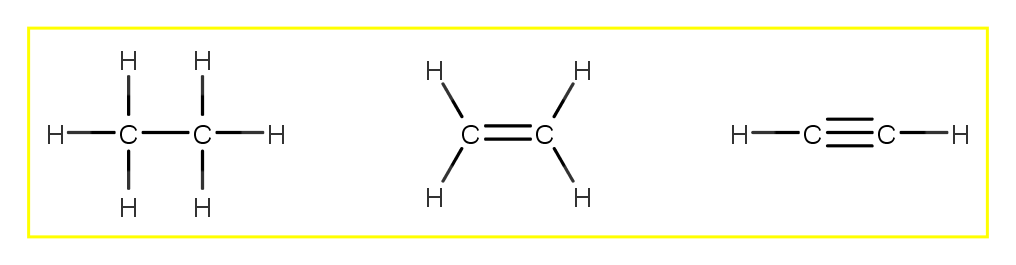
Part C Draw The Lewis Structures Of C2h6 Clutch Prep
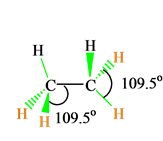
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Lewis Structure Of C2h6 Ethane Youtube