Is N2 Polar Or Nonpolar
Generally small symmetric molecules are nonpolar. There is no difference in electronegativities in the atoms of this molecule.
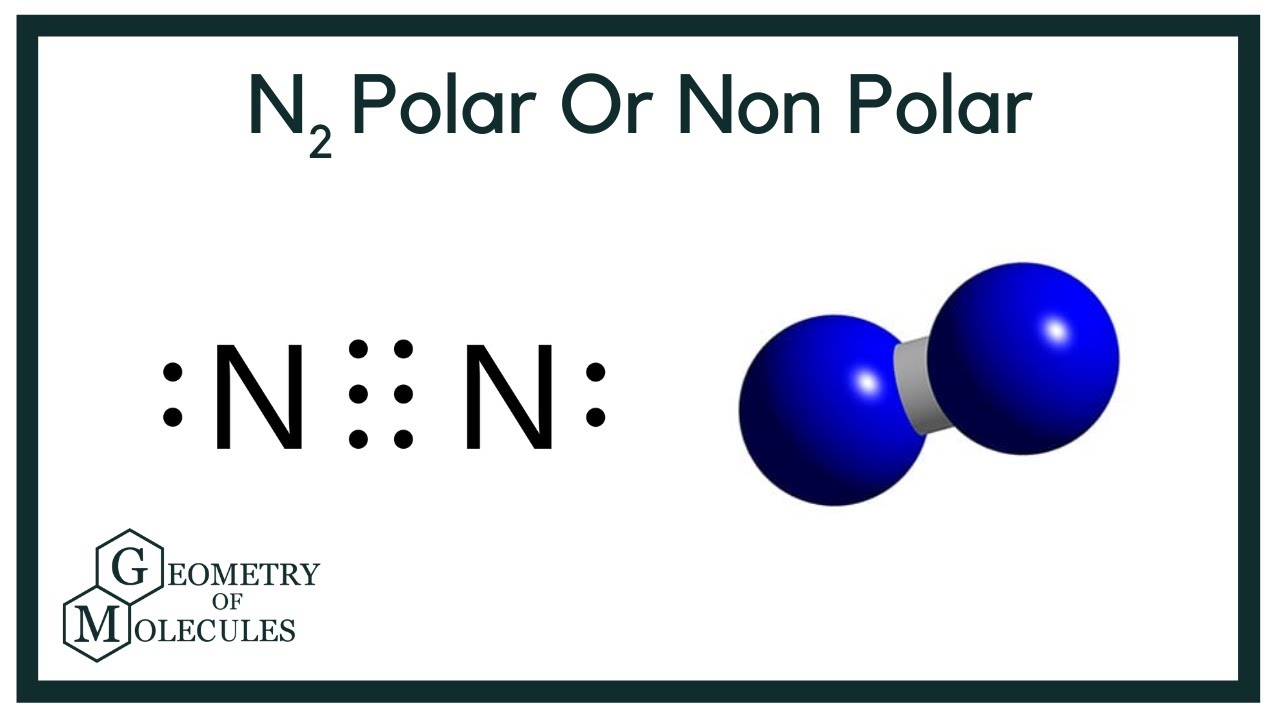
Is N2 Polar Or Nonpolar Nitrogen Polarity Explained Geometry Of Molecules
Is N2 linear or bent.

Is n2 polar or nonpolar. Why is CH2Cl2 dipole-dipole. A nitrogen atom has five electrons in its outermost electron shell. However there is still a small dipole moment so the molecule has dipole-dipole forces.
The answer must be letter c. Hence two nitrogen atoms bond with each other by sharing three electrons with each other. Each nitrogen in N2H4 has four pairs of electrons around it a lone pair a single bond to the other nitrogen and a single bond to each of the hydrogens.
We first look at. VSEPR predicts a tetrahedral geometry for the electron pairs. The N2 Lewis structure indicates that the N2 molecule is perfectly symmetric.
List molecules polar and non polar. What shape is icl5. The I-Cl bond is polar covalent.
Diatomic nitrogen N2 is non-polar since both sides of the molecule attract electrons more or less equally resulting in a non-polar molecule. There is no difference so there is no polarity. N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule.
In the molecule n2 both ends are the same. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. Since N2 is a symmetric molecule without a net electric dipole moment N2 is not polar.
Example molecules non polar Toluene Gasoline Helium He Neon Ne Krypton Kr Xenon Xe Hydrogen H2 Nitrogen N2 Oxygen O2 Carbon Dioxide CO2 Methane CH4 Ethylene C2H4 List molecules polar and non polar Molecules polar SCN- Thiocyanate HCO3- Bicarbonate BrCl3 Bromine Trichloride HCO3-1 AsCl3 Trichloroarsine OCl2 NO2Cl Nitryl chloride. How many nonpolar covalent bonds are in N2. Ill tell you the polar or nonpolar list below.
N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. Nitrogen Dioxide NO2 Polar or Nonpolar Basis of Characteristics NO2 is a polar molecule and the Oxygen atom closest to negative side as the electronegativity of Oxygen 344 is comparatively greater than Nitrogen 304 so that Nitrogen has a partial positive charge and Oxygen has a partial negative charge established within the molecule. Admittedly it is not very polar because ΔEN is only 050 corresponding to about 6 ionic character.
NO2 Nitrogen dioxide is Polar. Answer Expert Verified How many nonpolar covalent bonds will there be in N2 molecule. Nitrogen is a diatomic molecule N2 NN and has a net dipole moment of zero.
In CH2Cl2 the Cl pulls much more on the Carbons electrons since it has higher electronegativity than the Hydrogens which sets up a dipole moment ie. It has zero dipole moment. The tugs from each nitrogen on the bonding electrons are the same and opposite.
Because N2 molecules are nonpolar the intermolecular forces between them are dispersion forces also called London forces. The gives a net dipole moment of zero and a nonpolar molecule. Of course that means that nitrogen is nonpolar.
Answer N2 Nitrogen is Nonpolar What is polar and non-polar. A polar molecule has a net electric dipole moment. Nitrogen molecule is a non-polar covalent molecule.
Two N atoms in nitrogen molecule have zero electronegativity difference. Because we will be having 3 non-polar covalent bonds there in N2 molecule or Nitrogen molecule. Summing up everything we stated above we can say N2 or Nitrogen gas is a nonpolar molecule because there is no net dipole moment in the molecule as both the atoms are identical in nature.
N2 is non polar because is has no dipole moment ie it is completely even on both sides of the molecule o2 in. Dipole moment is zero in CO2 molecules and that is why it becomes a non-polar compound. Hcl is polar because ionic compounds are always polar and it has a net dipole moment.
N2 is nonpolar. The bond pairs of electrons are equally distributed between two N atoms. Molecular Geometry and Polarity.
The N-N bond is non-polar but the N-H bonds are polar hydrogen has a lower electronegativity than nitrogen has. N2 is a chemical formula for Nitrogen gas and in todays video we help you determine if this molecule is polar or nonpolar. As a result both atoms have equal electronegativity and share an equal proportion of charge and the overall molecule result in a net-zero dipole moment making it a nonpolar molecule.

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar
Is O2 Polar Or Non Polar Oxygen Gas Youtube

Is N2 Polar Or Nonpolar Techiescientist
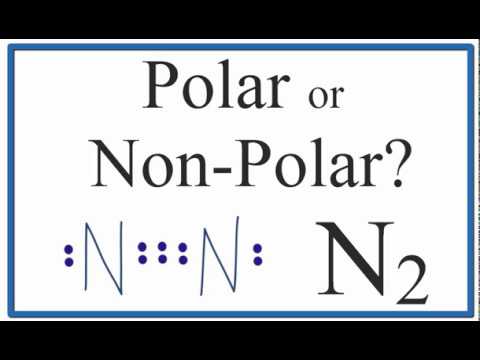
Is N2 Polar Or Non Polar Nitrogen Gas Youtube
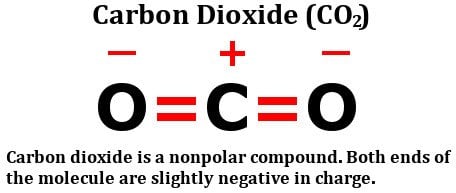
Is Carbon Dioxide Co2 Polar Or Nonpolar Science Abc
Is H2 Polar Or Non Polar Youtube

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Is Cl2 Polar Or Non Polar Chlorine Gas Youtube

Polar And Nonpolar Molecules Youtube

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula
Polar And Non Polar Molecules Vce Chemistry

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Is N2 Polar Or Non Polar Nitrogen Gas Youtube

Bond Polarity Chemistry For Non Majors

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples Covalent Bonding Chemical Bond Study Chemistry

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules

Is N2 Polar Or Nonpolar Techiescientist