Cocl2 Lewis Structure Molecular Geometry
A double bond represents a slightly larger electron domain which disrupts the ideal 120 CI-C-Cl bond angle making it smaller. Two electron pairs linear arrangement.
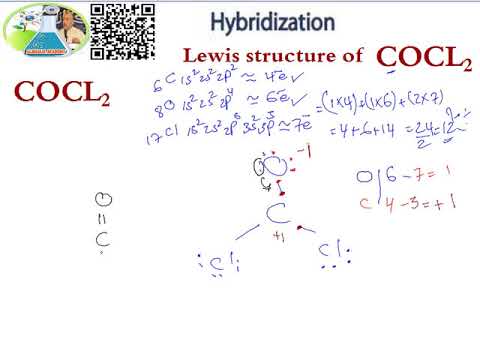
Lewis Structure Hybridization Cocl2 Youtube
Lewis Structure show all resonance structures if applicable Molecular Shape.

Cocl2 lewis structure molecular geometry. Yes No Molecular Polarity. COCl2 Total of Valence Electrons. D Sketch the molecule beginning with the central.
Lewis Structure show all resonance structures if applicable Molecular Shape. 5 rows CoCl2 Lewis Structure. Please label both geometries.
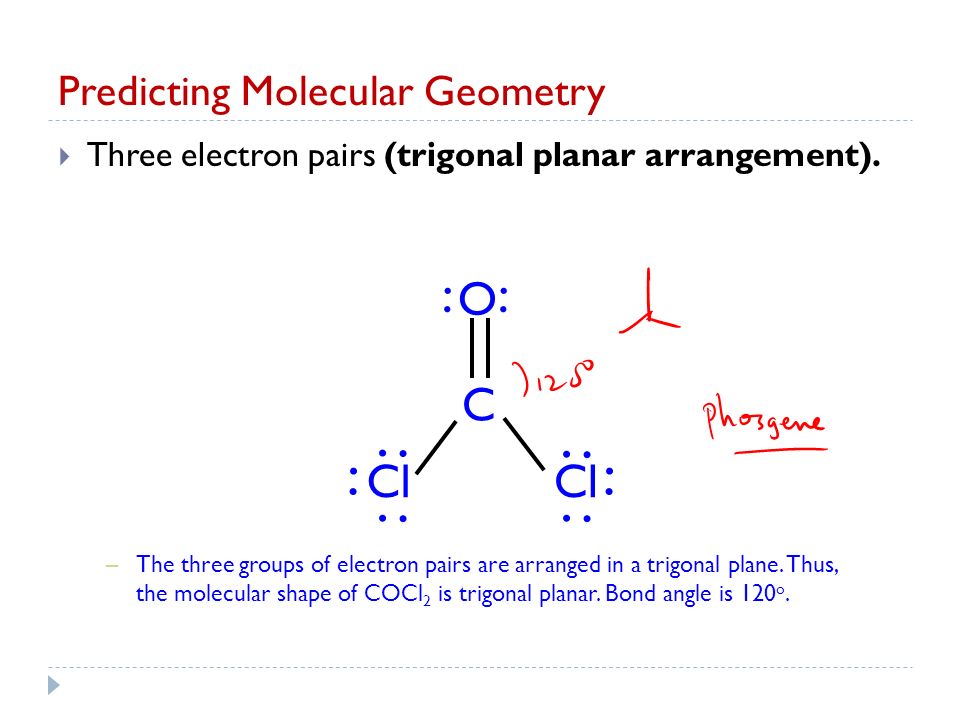
Thus the molecular shape of COCl2 is trigonal planar. Moreover there exist many lone pairs which do not alter the molecular geometry but make the molecule polar. The Lewis structure of a compound helps predict many of its.
Get the detailed answer. Apply VSEPR notation A X E ANumber of central atoms XNumber of surrounding atoms E Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3. Any polar bonds in the molecule.
The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar. How many sigma and pi bonds are present. You have two double bonds or two electron groups about the carbon atom.
The Lewis structure of the CoCl2 molecule shows that there is a double bond between the O and the central C atom. After drawing bonds the lewis structure looks like this. Check me out.
Use lewis structure guidelines to draw the lewis structure of COCl2. Answered 3 years ago Author has 431 answers and 9085K answer views. We have two single bonds between carbon and each of the two oxygen atoms.
3 A Draw the Lewis structure for phosgene COCl2. It can also be shown in the below image. In the cocl2 lewis structure carbon is less electron electronegative than oxygen and goes in the center of the lewis structure note that hydrogen atoms always go on the outside.
Both CCl bonds are polar due to the difference in electronegativity of C and Cl. The bond angle is 180o. Arrangement - AX3 with 3 bonding pairs no lone pairs on the central atom.
3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable. Is the CCl bond in COCl2 polar or. Here We have each of the oxygen having octet configuration We have carbon having octet configuration.
What Is The Lewis Structure For COCl2 And What Is The Molecular Shape. Any polar bonds in the molecule. A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure PhosgeneFor the COCl2 structure use the periodic table to find the total number of.
C Using Valence Bond theory what is the hybridization of the carbon atom. Thus according to the VSEPR model the bonds are arranged linearly and the molecular shape of carbon dioxide is linear. AX 3 has trigonal planarl shape.
We have one double bond between C and one O atom. 3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable. Starting with its Lewis structure the C_2Cl_2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of.
The three groups of electron pairs are arranged in a trigonal plane. One needs to know the Lewis structure in order to understand the molecular geometry of any. B Using VSEPR Theory what is the electron geometry and molecular geometry of the compound.
What is the molecular shape of COCl2. Draw the Lewis structure for COCl2 including lone pairs. The Lewis structure of the COCl2 molecule shows that there is a double bond between the O and the central C atom.
What Is The Lewis Structure For COCl2 And What Is The Molecular Shape. Use VSEPR table to find the shape. This problem has been solved.
Look at this Lewis Structure. Carbonyl fluoride COF2 is a toxic and inflammable compound whose Lewis structure determines the presence of a double bond between the carbon and oxygen atoms and single bonds between the carbon and fluorine atoms. Yes No Molecular Polarity.
A double bond represents a slightly larger electron domain which disrupts the ideal 120 Cl-C-Cl. COCl2 Total of Valence Electrons. In the lewis structure for cocl2 there are a total of 24 valence electrons.

The Lewis Structure Of Compound Cocl2 Contain How Many Lonw Pair Of Electrons
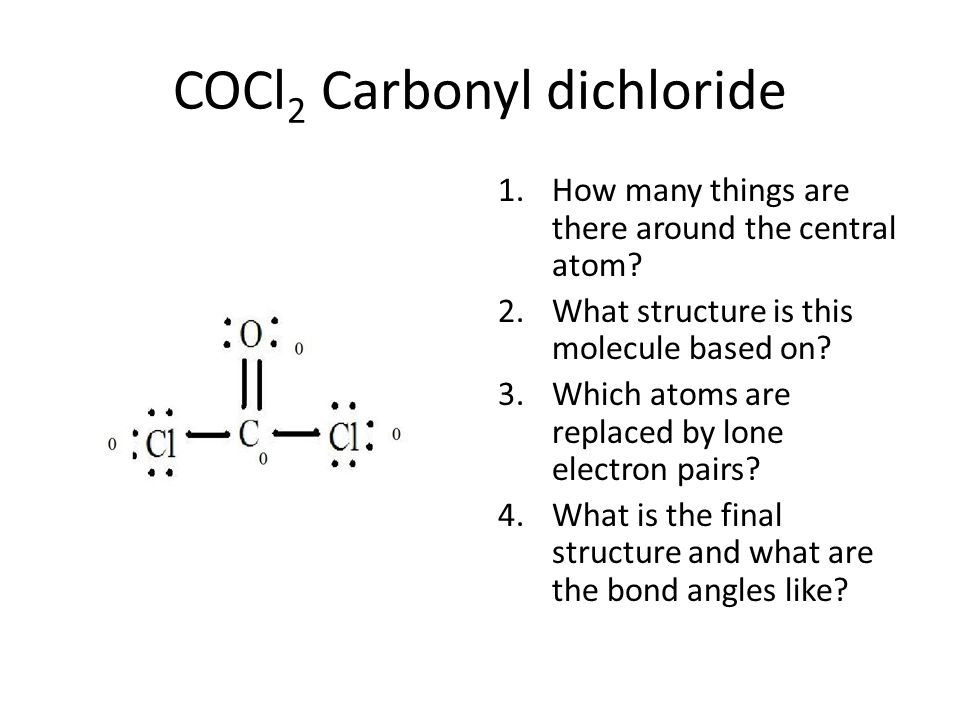
Covalent Bonding Shapes Valence Sheell Electron Pair Repulsion Ppt Video Online Download
Carvone Bucky Ball Molecular Geometry Chapter 8 Part Ppt Video Online Download

Oneclass Draw The Lewis Structure For Cocl2 Including Lone Pairs What Is The Molecular Shape Of Co

How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep
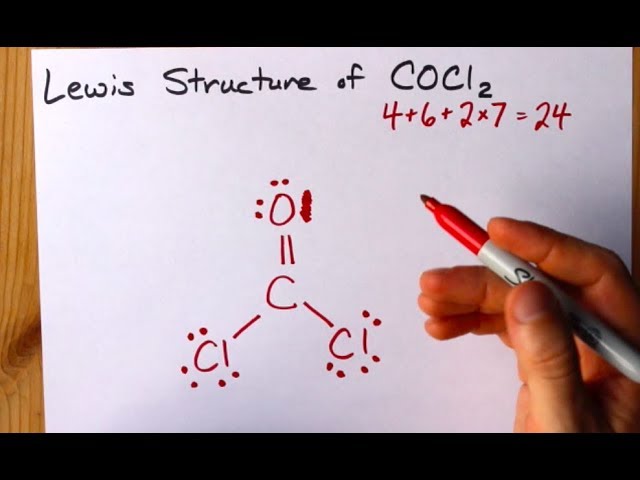
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
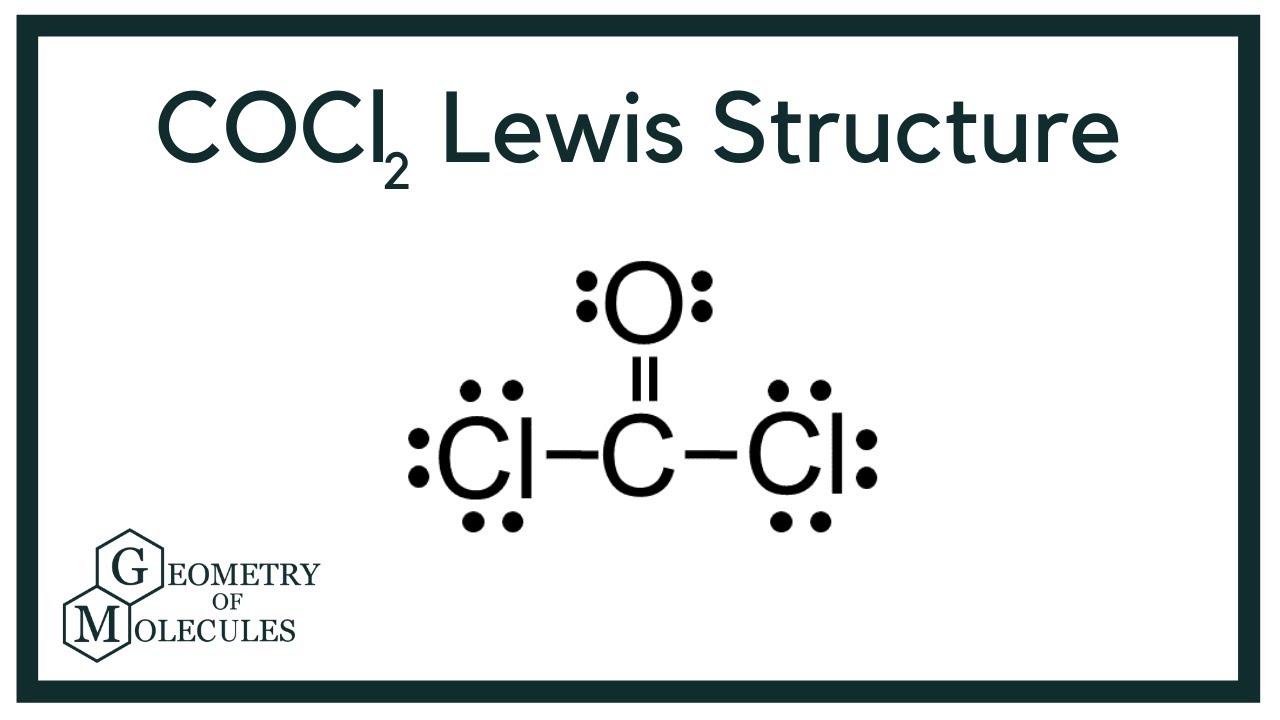
Cocl2 Lewis Structure Phosgene Youtube
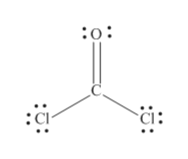
Answered What Is The Molecular Shape Of Coc12 Bartleby

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Cocl2 Phosgene Molecular Geometry Bond Angles And Electron Geometry Youtube
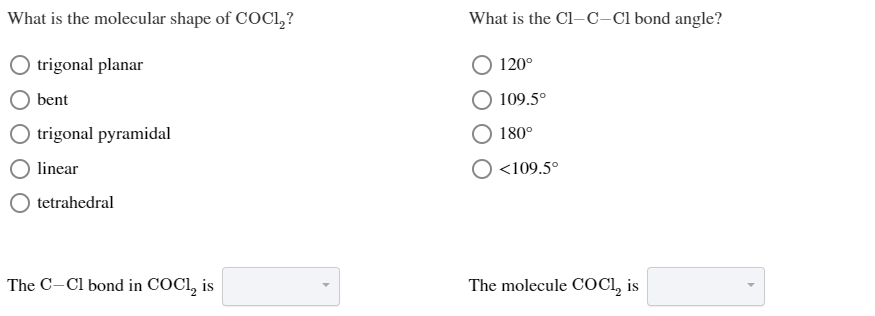
Answered What Is The Molecular Shape Of Coc12 Bartleby

What Is The Molecular Shape Of Cocl2 Quora
