Is H3o+ Polar Or Nonpolar
Get the detailed answer. Is H3O polar or non-polar.
H3O is an important compound in Acid-Base chemistry and is considered an acid.

Is h3o+ polar or nonpolar. HClO HYPOCHLOROUS ACID Polar. Is hydronium ion H3O polar or non-polar. A simple way to know if its polar or nonpolar is to draw the lewis dot structure and use VSEPR.
Is POCl3 polar or nonpolar. Is hydronium ion H3O polar or non-polar. Furthermore the positive charge on the oxygen causes this molecule to be a cation which therefore automatically exhibits polar qualities.
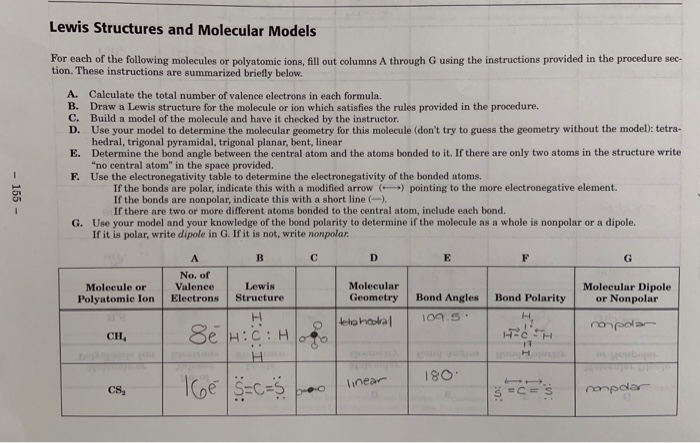
If it is not write nonpolar - 155 - Molecule or Polyatomie Ion No. So H3O has 3 polar bonds and the overall molecule is polar too. Answer H3O Hydronium is Polar What is polar and non-polar.
So 12 is polar bond. So H3O has 3 polar bonds and the overall molecule is polar too. Another question on Chemistry.
HBr HYDROBROMIC ACID Polar. So 12 is polar bond. Try structures similar to H 3 O for more practice.
For H3Oprovide a Lewis structure predicted VSEPR molecular geometry bond angle and indicate whether the compound is polar non polar or polyatomic ion. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Watch the video and see if you missed any steps or information.
H3O is a polar molecule due to the existence of a pair of lone pair electrons on top of the molecule causing electron-electron repulsion. Phosphoryl Chloride or Phosphorus Oxychloride is a polar molecule due to the uneven distribution of valence electrons in the molecule and a net dipole moment in it. Hco2h formic acid Polar.
H3O Hydronium is Polar Ill tell you the polar or nonpolar list below. Free unlimited access for 30 days limited time only. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
A simple way to know if its polar or nonpolar is to draw the lewis dot structure and use VSEPR. Get the detailed answer. If the difference is from 0-04 the bond is nonpolar but if its from 05-19 the bond is polar.
H3PO4 Phosphoric acid Polar. It is helpful if you. So 12 is polar bond.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Learn to determine if H2S Hydrogen sulfide is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis St. My teacher taught me that when considering the polarity of a molecule you first look at the kind of bond that exists.
So H3O has 3 polar bonds and the overall molecule is polar too. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. If it is polar write dipole in G.
Try to draw the H 3 O Lewis structure before watching the video. For Example If The Molecule Were HCl And You Decided The Hydrogen Atom Was Closest To The Negative Side Of The Molecule Youd Enter. Question Is H3PO4 Phosphoric acid polar or nonpolar.
If the difference is from 0-04 the bond is nonpolar but if its from 05-19 the bond is polar. HCN Hydrogen cyanide Polar. List of Lewis Structures.
Due to the asymmetry seen in the molecules shape the dipole moments are not nullified making this molecule a polar molecule. HF Hydrogen fluoride Polar. Answer H3PO4 Phosphoric acid is Polar What is polar and non-polar.
If polar draw the molecular dipole. The oxygen is sp3 hybridized with the lone pair in one of the sp3 orbitals making the geometry of the compound trigonal pyramidal same as NH3. This makes the molecule have an oxygen end and a hydrogen end making it polar.
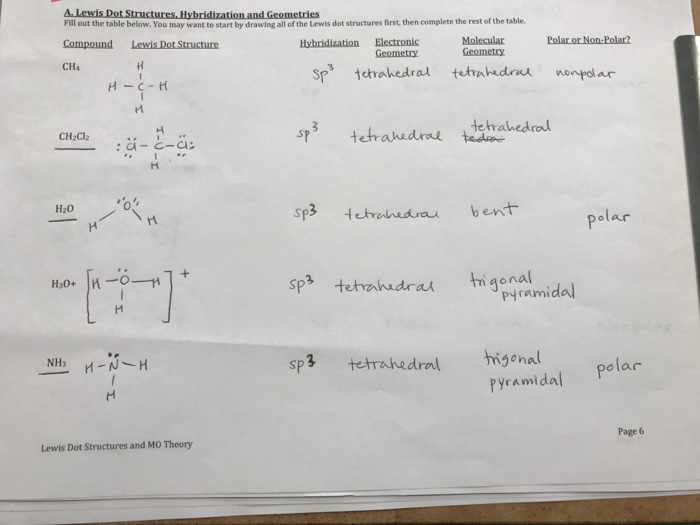
Of Valence Electrons Lewis Structure Molecular Geometry Bond Polarity Molecular Dipole or Nonpolar Bond Angles 1095 tetrahedral non pola CH Se HCH. Question Is H3O polar or nonpolar. A simple way to know if its polar or nonpolar is to draw the lewis dot structure and use VSEPR.
A nonpolar solvent will dissolve in a nonpolar solvent but not a polar solvent. Do the following have resonance structuresisomers if so what are they. State whether they are polar or non-polar.
This results in a bent structure that leads to an unequal distribution of charge within the molecule. HCl hydrogen chloride Polar. Log in Sign up.
If the difference is from 0-04 the bond is nonpolar but if its from 05-19 the bond is polar.

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
Lewis Dot Structures Hybridization Geometries Chegg Com

Decide Whether Each Molecule Or Polyatomic Ion Is Chegg Com
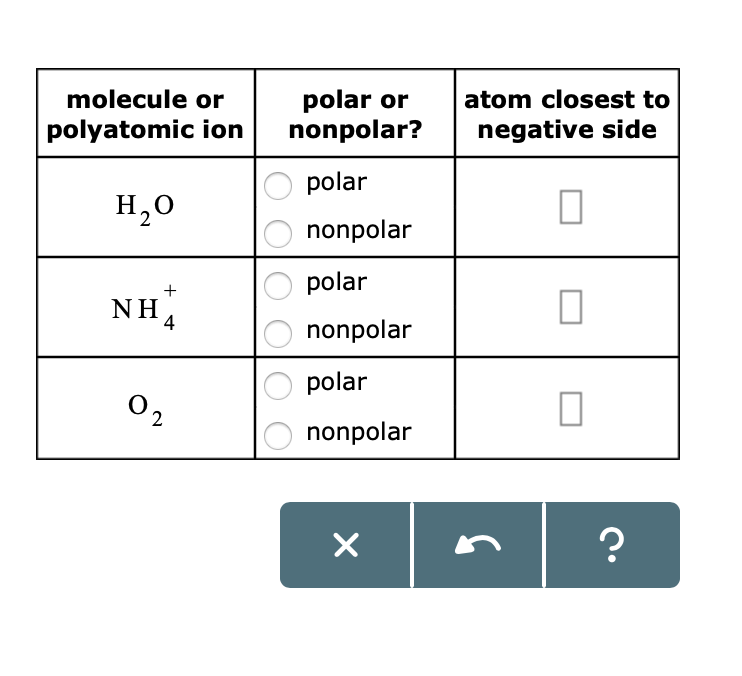
Polar Or Atom Closest To Polyatomic Ionnonpolar Chegg Com

Is H3o Polar Or Non Polar Hydronium Youtube
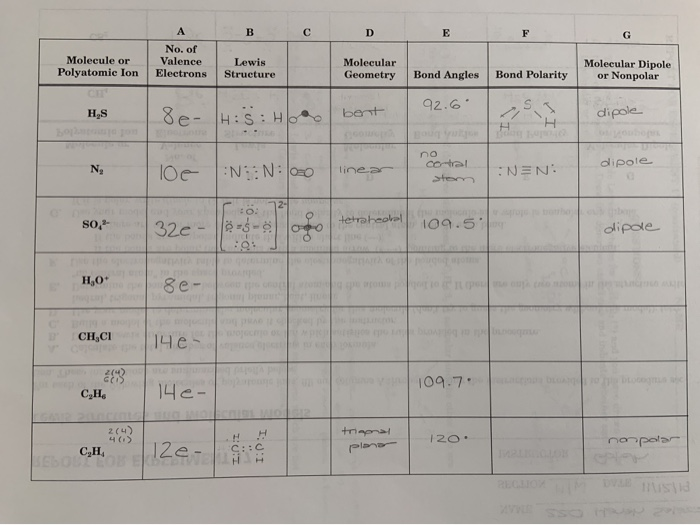
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

Is H3o Polar Or Non Polar Hydronium Youtube

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
What Is The Structure Of H3o Quora

Which Compound Is Formed By A Covalent Bond Bacl2 Chegg Com

Is H3o Polar Or Non Polar Hydronium Youtube

The Molecule Xf3 Has A Dipole Moment Is X Clutch Prep
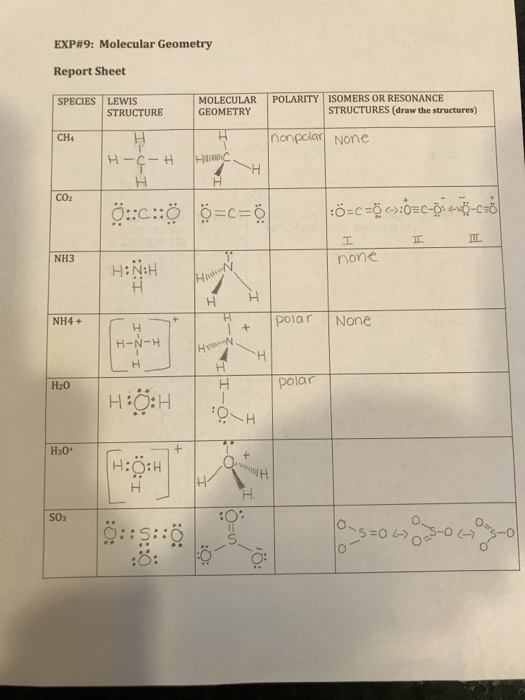
I Have To Draw The Isomers Or Resonance Structures I Chegg Com

Decide Whether Each Molecule Or Polyatomic Ion Is Chegg Com

Is Ch2o Polar Or Nonpolar Methanal Or Formaldehyde Youtube
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com