Does Pcl5 Have Resonance Structures
The bonds mature in 11 years and have a 1000 face value. For HNO3 in order to.

Lewis Structure Of Pci5 Brainly Ph
It is still a valid resonance structure octets do not have to be complete for it to be valid.

Does pcl5 have resonance structures. The Lewis dot structure. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis structure for the molecule. Pcl5 Resonance Structures Free PDF eBooks.
So PCl3 does not exhibit resonance. So this molecule is polar. With this model we can draw a series of resonance structures as shown below for PF5.
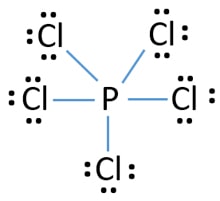
In the PCl 5 Lewis structure Phosphorus P is the least electronegative so it goes in the center. Sp3 Molecular geometry of PCl3 is trigonal pyramidal with asymmetric charge distribution on central atom. When we examine the Lewis structure of PCL3 we can see that each chlorine atoms have 3 lone pairs and all of them must have 8 electrons around it.
Does PCl3 have resonance. There is only one structure needed to represent SeI2. What is the Lewis structure of PCL3.
Thus PF5 has net four covalent bonds and one ionic bond. A step-by-step explanation of how to draw the PCl5 Lewis Dot Structure Phosphorus pentachlorideFor the PCl5 structure use the periodic table to find the t. Check me out.
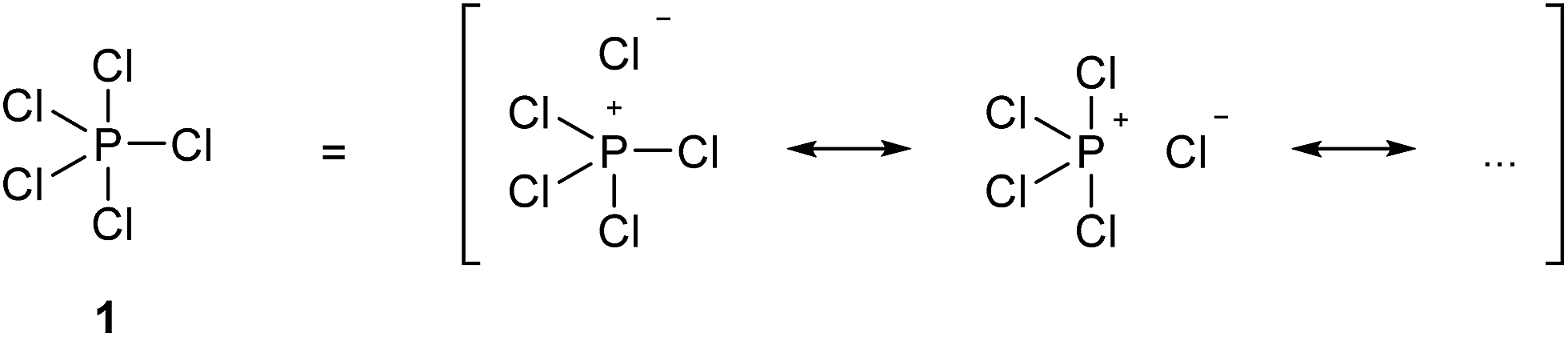
ReadDownload File Report Abuse. Draw the Lewis dot structures and resonance structures for the following. The net sum of valid resonance structures is defined as a resonance hybrid which represents the overall delocalization of electrons within the molecule.
What is the name of pcl5. In compounds SF6 IOF5 IF5 BrF5 XeF4 PF6 etc the center atoms have 12 electrons. Give the Lewis structure for PCl5.
Resonance structures are used when one Lewis structure for a single molecule cannot fully describe the bonding that takes place between neighboring atoms relative to the empirical data for the actual bond lengths between those atoms. Hence lewis structures of two molecules are different and their resonance structures are also different. There is no NO 2 ion.
It does mean it will not contribute much to the overall structure of the molecule but that resonance structure does show us why carbonyl carbons are reactive towards nucleophiles. They are different because total valance electrons of two molecules are different. He bonds issued by Stainless Tubs bear a 6 percent coupon payable semiannually.
A molecule that has several resonance structures is more stable than one with fewer. Hence it does not exhibit resonance. Posted on November 09 2016.
Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table. When drawing the Lewis structure for PCl5 five chlorine Cl atoms are bonded to the central atom phosphorous P. Resonance structures are used when one Lewis structure for a single molecule cannot fully describe the bonding that takes place between neighboring atoms relative to the empirical data for the actual bond lengths between those atoms.
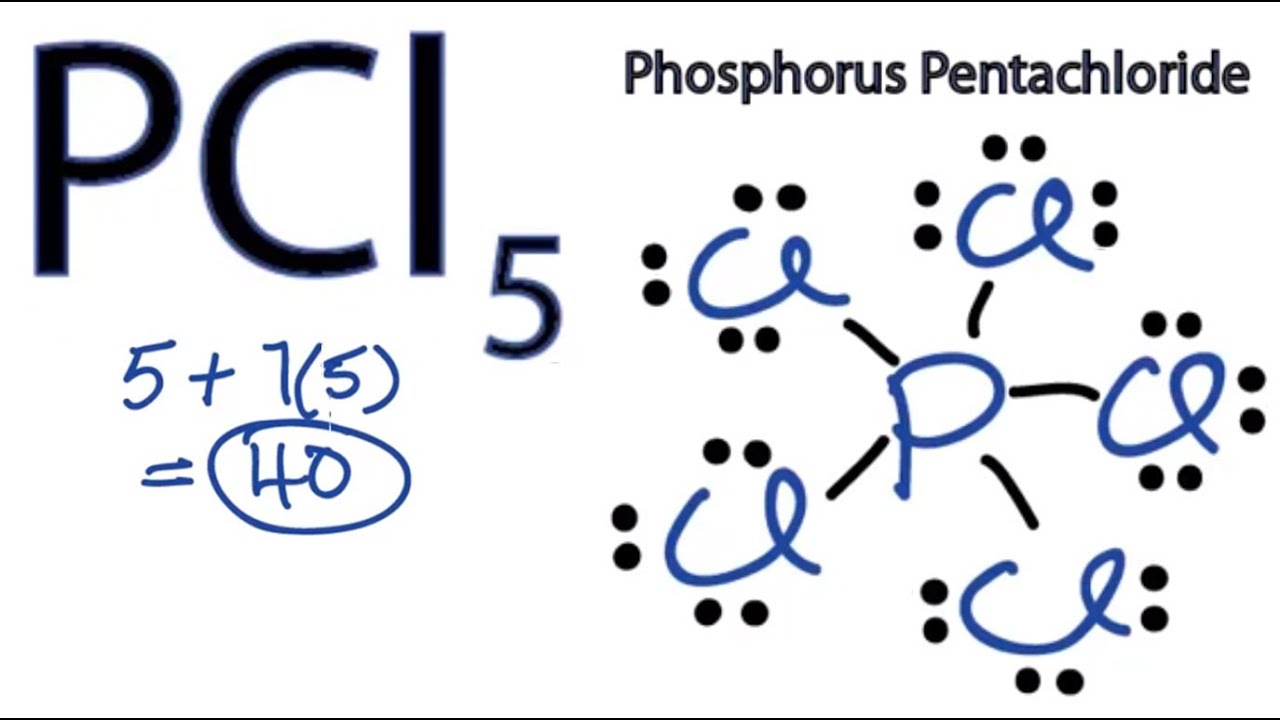
Drawing the Lewis structure. In the Lewis structure for PCl 5 there are a total of 40 valence electrons. It is not one of the structures but all of them superimposed.
Five pairs will be used in the chemical bonds between the P and Cl. By signing up youll get thousands of step-by-step solutions to your homework questions. Any extra bonds are ionic in character with the extra electrons assigned to the outer atoms.
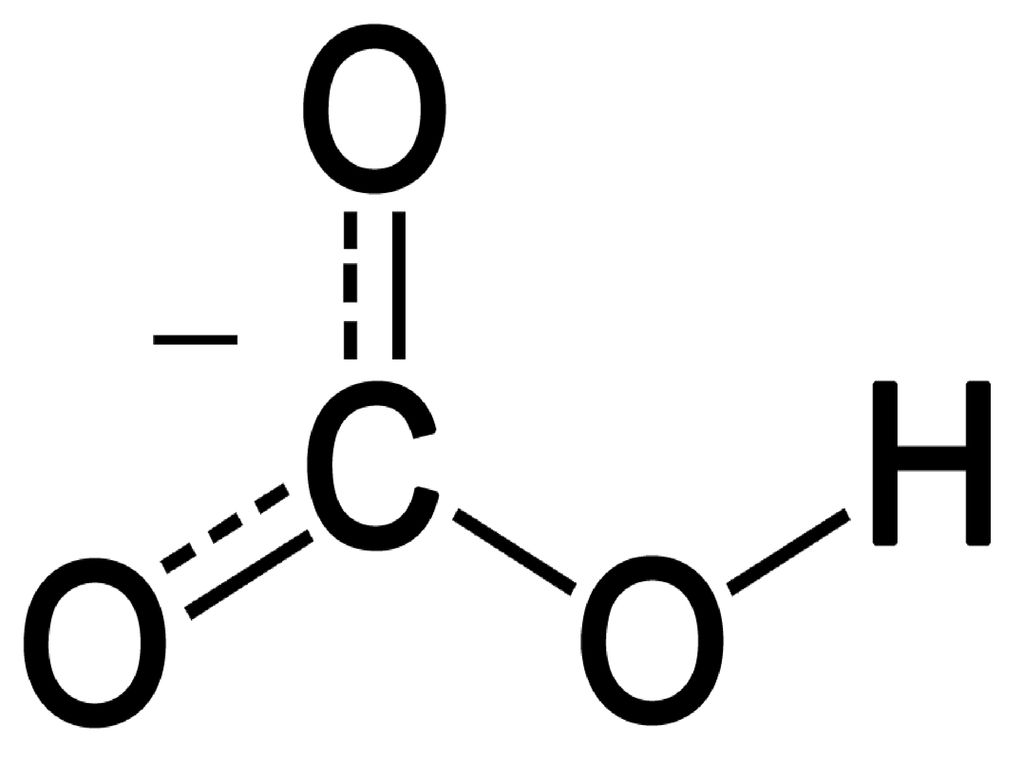
I would like to know. Does carbonate ion have any resonance structures. There are a total of 40 valence electrons in the PCl5 Lewis structure.
Carbon has 4 valence electrons each oxygen has 6 valence electrons and there are 2 more for the 2 charge. The Carbonate CO23 Ion Unlike O3 though the actual structure of CO32 is an average of three resonance structures. PCl3 Phosphorus Trichloride.
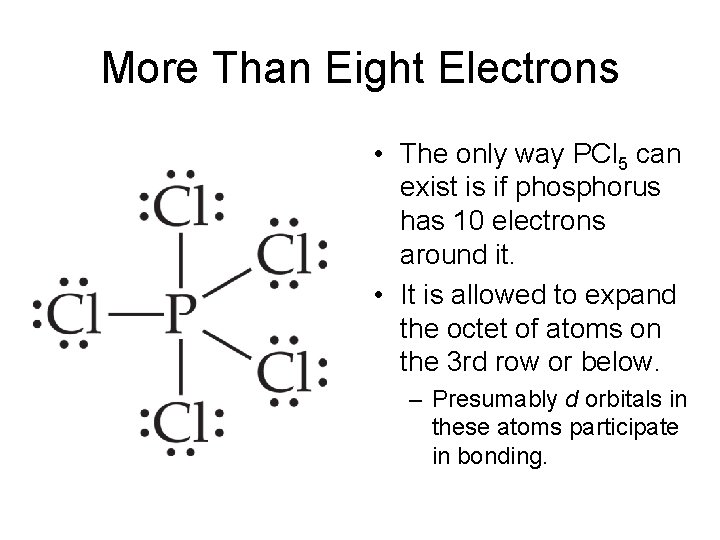
As you draw them keep in mind that some of the resonance structures may not. In compounds PF5 PCl5 SF4 ClF3 XeF2 and I3 the center atoms have 10 electrons instead of 8. A molecule that has several resonance structures.
OCl2 does not have resonance structures and does not exhibit resonance. The net sum of valid resonance structures is defined as a resonance hybrid which represents the overall delocalization of electrons within the molecule. Does PF5 have resonance structures.
In this case they do not exceed the octet rule. So PCl3 does not exhibit resonance. Is NO 2 a resonance structure of NO 2-No.
Currently the bonds sell for 989. In PCl3 there are no pi electrons there are no double bonds. Lewis Structures of Compounds Drawing correct Lewis structures.
This means that it can hold more than 8 valence electrons. Postby Julie Nguyen 1B Mon Jul 11 2016 728 am. Some hints are given.
Does HNO3 have resonance structures. Phosphorus pentachloride PCl5 as exception to octet rule. Check the lewis structure of NO 2 and resonance structures of NO 2.
A Quantitative Definition Of Hypervalency Chemical Science Rsc Publishing Doi 10 1039 C5sc02076j

What Is The Lewis Structure Of Pcl5 Quora
Molecular Geometry Ck 12 Foundation
Pcl5 Phosphorus Pentachloride Lewis Structure
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Draw The Lewis Structure For Methane Ch4 Ppt Video Online Download
Catalyst 1 Draw The Lewis Structure For Bcl3 2 Draw The Lewis Structure For Hcn 3 Draw The Lewis Structure For Pcl5 Ppt Download
Answer In Physical Chemistry For Anuj 84823
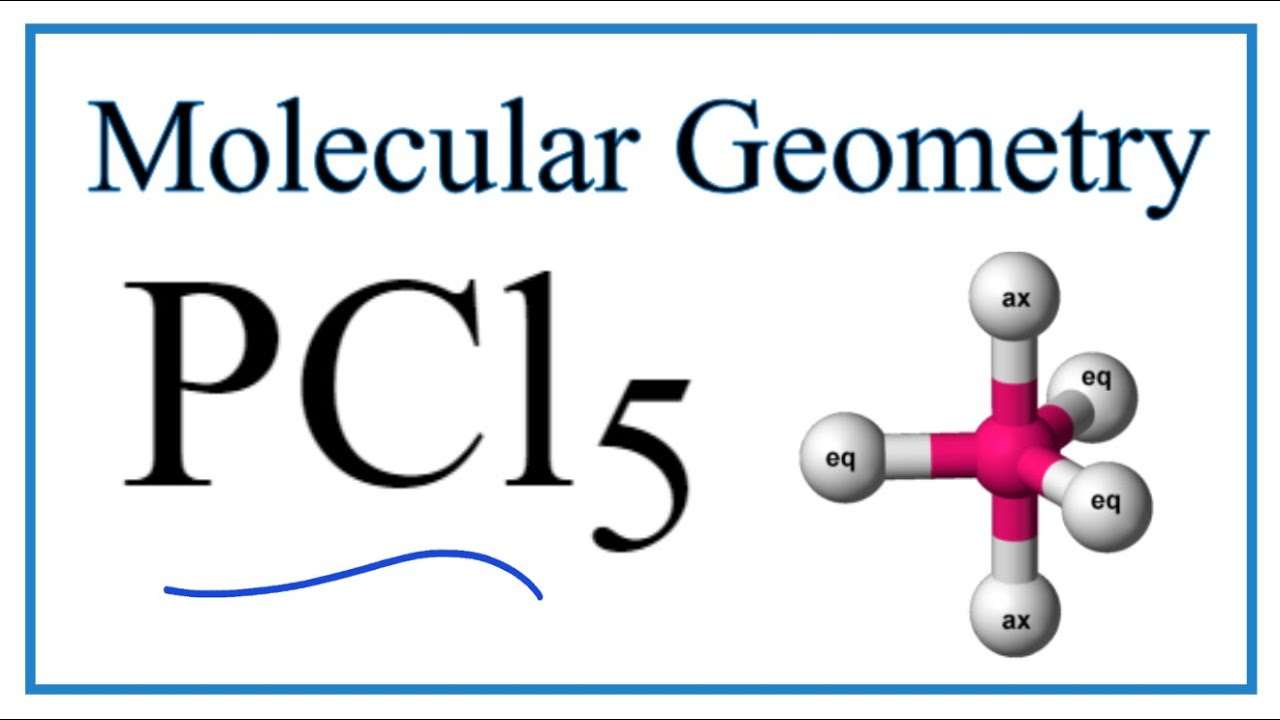
Pcl5 Phosphorous Pentachloride Molecular Geometry Bond Angles Youtube

Is Pcl5 Polar Or Nonpolar Techiescientist
Http Www Glimme Net Apchem Ch8 9 Bonding 3 Handout Pdf
What Is The Lewis Structure Of Pcl5 Quora

How To Determine The Lewis Structure For Pcl5 Quora
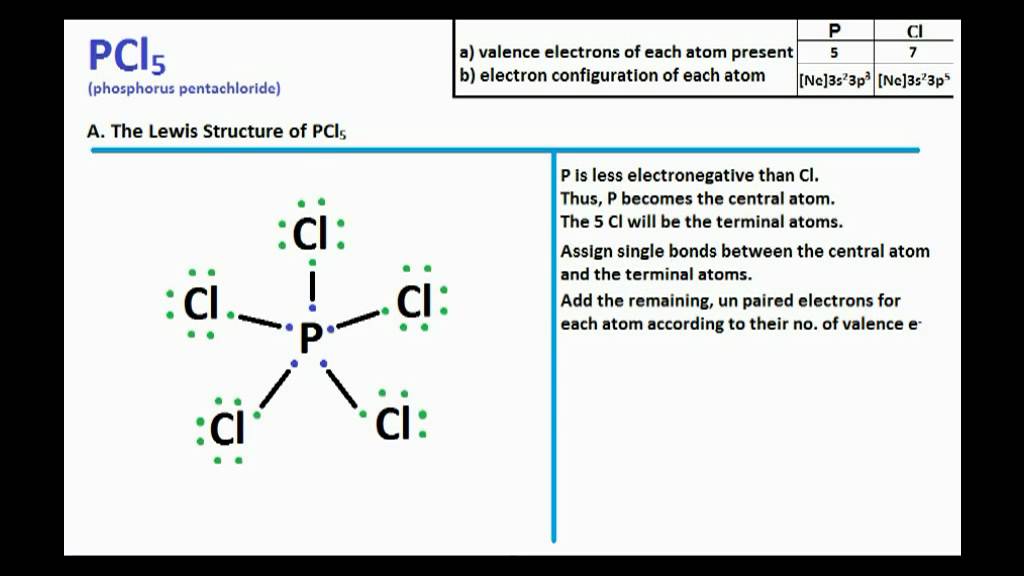
Pcl5 Lewis Structure And Molecular Geometry Youtube
Draw The Lewis Structure Of Pcl5 And Sf6 Sarthaks Econnect Largest Online Education Community

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
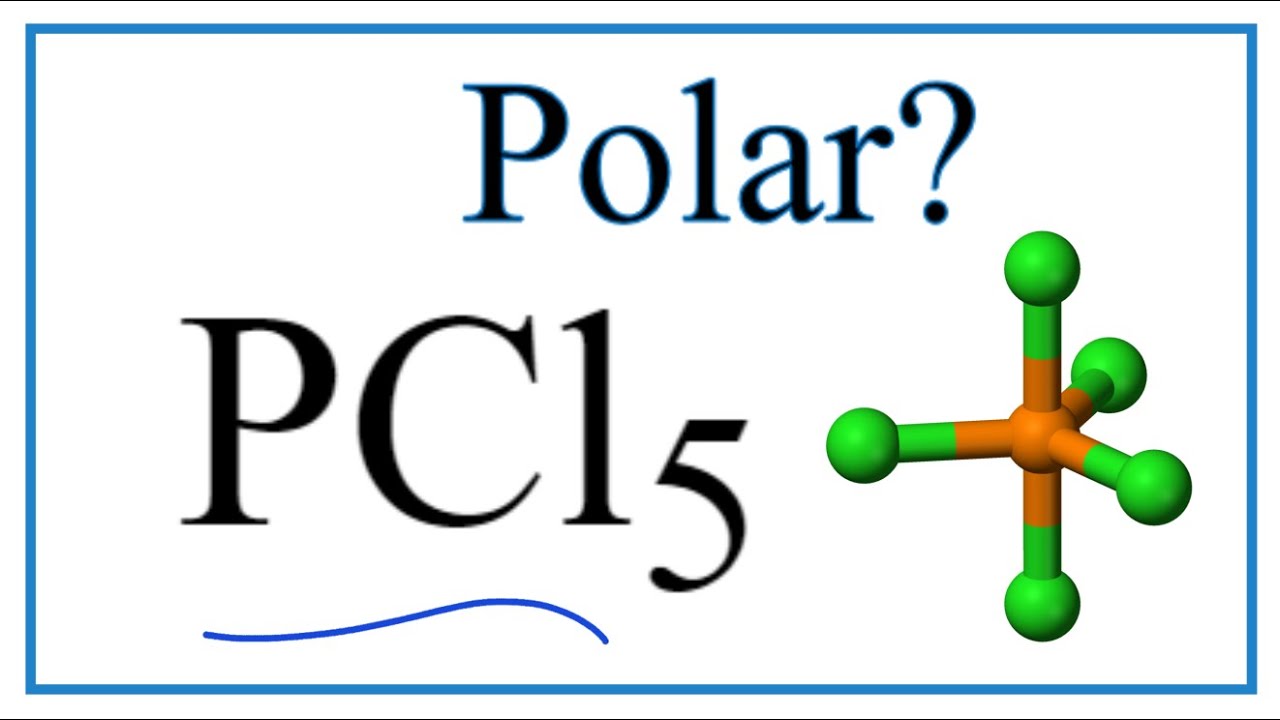
Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride Youtube