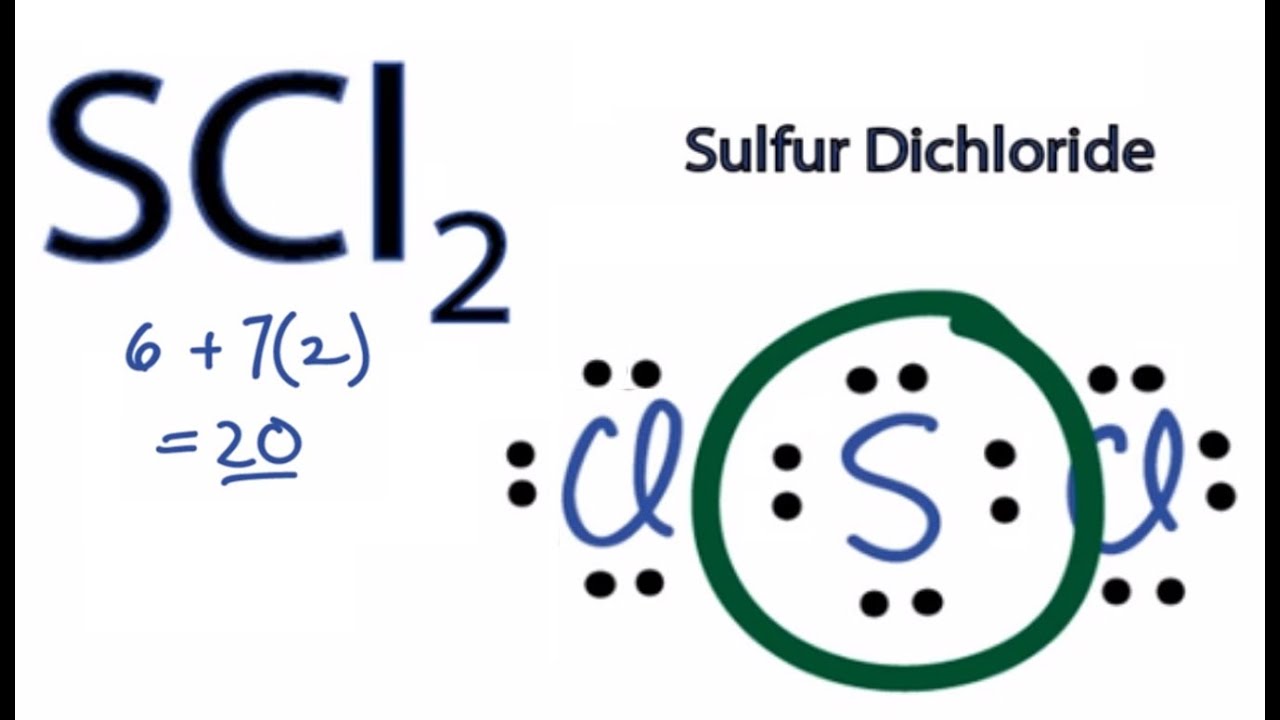
How Many Valence Electrons Does Scl2 Have
We know the details about this. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Khurak
Chemistry Multiple Choice 2.

How many valence electrons does scl2 have. And then well go around the outside and fill the octets for the Bromine until weve used 32 valence electrons. This preview shows page 16 19 out of 22 pages. A 1 B 2 C 4 D 7.
Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center. Metals are the elements which have a tendency to loose electrons and thus they form cations. So we have 8 10 12 and 32.
Total number of valence electrons for SF 2 6 72 as there are two atoms of Fluorine we will multiply the number by 2 6 14 20 valence electrons. How many valence electrons of beryllium ion have. 1 N 1 O 15 16 11.
There are 2 types of elements in the periodic table. For NO the skeleton structure is N-O. The Cl is sp3 hybridized and the molecule is angular.
This results in 4 electrons used. A I B F C H D He. Total number of valence electron available for SCl2 lewis structure 6 72 20 valence electrons.
For the SCl2 Lewis structure we have a total of 20 valence electrons. In this case both the valence and valence electrons of beryllium are 2. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom.
Cl is in the center bonded to the 2 Oxygen atoms. There are 20 valence electrons on the chlorite. A SCl2 B KCI C HCI D S2Cl2.
How many valence electrons does an atom of any halogen have. 8 valence electrons What is the electron dot structure for chlorine. Question How many valence electrons does magnesium have.
We know the details about this. Answer Magnesium has just two valence electrons and 12 electrons. After the electron configuration the last shell of the beryllium atom has two electrons.
In this case both the valence and valence electrons of boron are 3. Magnesium is classified as a metal. The valence electrons you have available are.
The formula for the compound formed between calcium and sulfate ion is which of these. The two C-O single bonds and the CO double bond. The total number of valence electrons will be 28 7 from each of the three chlorine atoms and 7 from the bromine atom.
Each bond uses up two valence electrons which means we have used a total of six valence electrons. They can determine the number of kernel electrons and the number of valence electrons due to the bonds they form for example Sulfur is more likely to form ions with the Alkaline earth metals and form different covalent bonds. For example when two chlorine atoms each with 7 valence electrons come together to form a diatomic chlorine molecule the Lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electronsLewis Dot Structures.
One element that has 7 valence electrons is _____. Sulfur has six valence electrons in its outer shell. Total number of valence electrons for SF 2 Valence electrons of Sulphur Valence electrons of Fluorine.
Fluorine has seven valence electrons. The elements that have 1 2 or three electrons in the last shell donate the electrons in the last. But these electrons are concentrated in three places.
Electronegativities of C and H are about equal. Which of these elements does not exist as a diatomic molecule. The elements that have 1 2 or three electrons in the last shell donate the electrons in the last shell during bond formation.
Does ICl5 have a dipole moment. Which one of the following compounds is not covalent. Well put 2 electrons between atoms to form chemical bonds and weve used 8.
Total valence electrons 14 6 20 S is the central atom and has two sets of bonding electrons - one set for each bond to Cl. The trial structure is You have 14 valence electrons in your trial structure. There are 8 electrons around each atom with two nonbonded electron pairs on the Cl and three nonbonded electron pairs on each Oxygen.
Sodium will loose 1 electron. So weve used all 32 valence electrons. Sulfur has 6 Valence electrons 2 in the first shell 8 in the second shell and 6 in the outermost layer third layer.
Chlorine atom shares one valence electron of Phosphorus to complete its octet. What element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3. The elements that form bonds by donating electrons are called cations.
Now if you look at the molecule every Chlorine atom has a complete octet as. The trial structure has three extra electrons.

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom
Bond Angle Of Scl2 Lewis Structures

Science Coverage Is Asf3 Polar Or Nonpolar In 2021 Molecular Geometry Polar Octet Rule

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization
Valence Shell Electron Pair Repulsion

Does Scl2 Have A Dipole Moment Clutch Prep

The Lewis Diagram For Scl2 The Electron P Clutch Prep
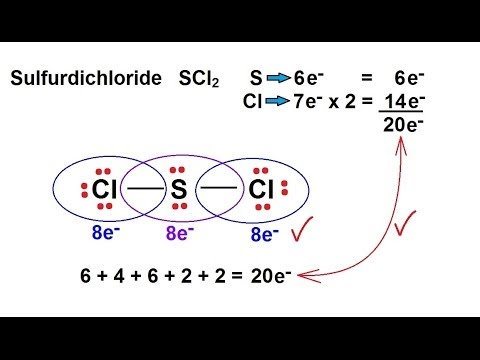
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Is Scl2 Polar Or Nonpolar Techiescientist

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons
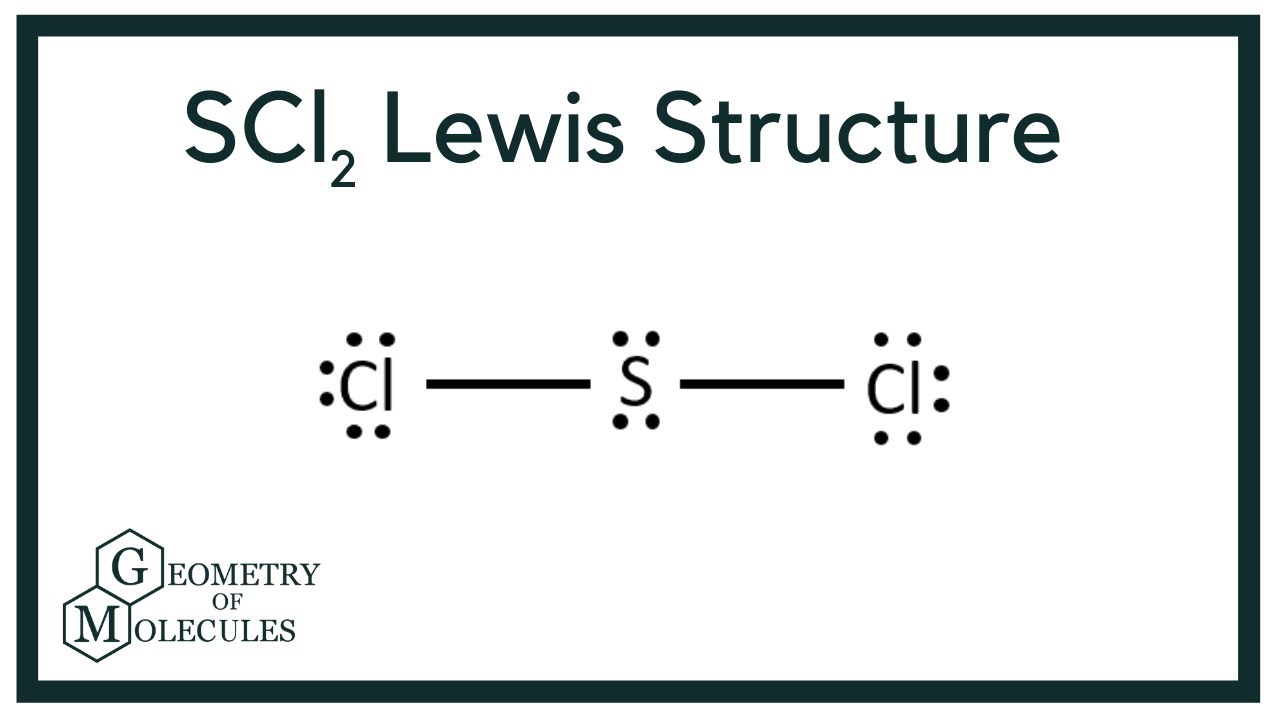
Scl2 Lewis Structure Sulfur Dichloride Youtube