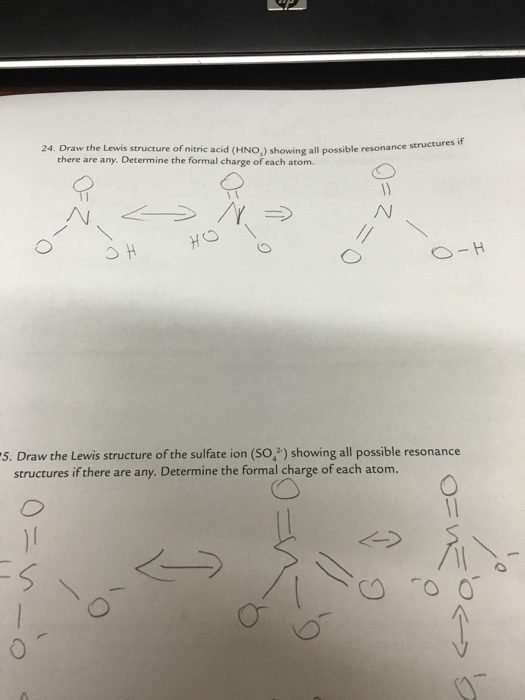
Lewis Structure Of Hno3 With Formal Charges
Remember the definition of a Lewis acid. Draw the lewis dot structure of H2SO4 show formal charges.
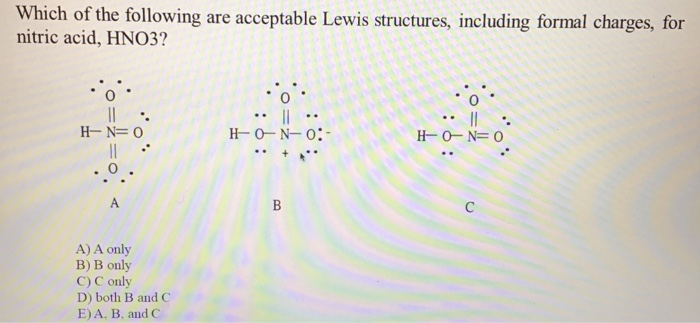
Which Of The Following Are Acceptable Lewis Chegg Com
Draw a correct Lewis structure for acetonitrile CH3CN.

Lewis structure of hno3 with formal charges. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. The nitrogen in HNO3 has a formal 1 charge and a formal double bond to one of its oxygens. Formal charge on an atom in a lewis structure.
Show how its stronger acid than HNO3. If the Lewis structure must have nonzero formal charges the arrangement with the smallest nonzero formal charges is preferable. Lone Pair e- Q Bond Pair e- 18 4 14.
Lewis dot formulae should and do reflect this neutrality. Labels can be used once more than once or not at all. Reason In Lewis representation the least electronegative atom occupies the central position in the moleculeion.
Cannot be fulfilled Lewis structures which have the least number of elements with sub-octet structures are preferred. A step-by-step explanation of how to draw the HNO3 Lewis Structure Nitric Acid. In this structure the central atom is nitrogen because it is in least number.
Both sulfuric and nitric acids are NEUTRAL species. Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign. Make the bonds between central atom and corner atoms.
Click hereto get an answer to your question Write lewis structure of the following compounds and show formal charge on each atom HNO3 NO2 H2SO4. After determining how many valence electrons there are in HNO3 place them around the central atom to. What Is The Correct Lewis Structure For Nitric Acid HNO3 Including The Formal Charges.
This is a pattern seen with many acids. HNO3 NO2 H2SO4. Lewis structure of nitric acid There is a NO bond in nitric acid lewis structure.
Part A Write formal charges for each atom in nitric acid HNO3 which has the Lewis structure shown. Drag the appropriate labels to their respective targets. Lewis structures in which all atoms have an octet doublet for hydrogen are preferred.
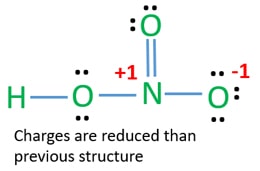
Formal charge on an atom in a Lewis structure total number of valence electrons in free atom total number of non-bonding lone pairs electrons 12 total number of bonding or shared electrons. One of the oxygen atoms has a formal negative charge and the nitrogen atom is quaternized and bears a formal positive charge. 1 Draw the Lewis structure of nitric acid HNO 3 that minimizes formal chargesAssign lone pairs radical electrons and atomic charges where appropriate 2 Calculate the electrons required.
In this case the formal charges will be closer to zero if you place a double bond beteween the Nitrogen atom and the Oxygen atom without the H attached. View Available Hints ResetHelp. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. Check the formal charges to be sure that each atom has a formal charge of zero.
A reasonable Lewis structure is H O N OO. Lewis Structure of NO 2 1-Q 5 2 x 6 1 18. Bone Pair e- 4.
HON HONo oz. See 11 answer is B only it is the only one with a charge. A I B II C III D IV E None Of These.
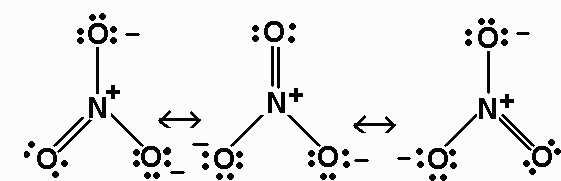
Generate a set of possible structures choose one that has a minimal number of formal charges. The HNO3 Lewis structure is best thought of as the NO3 with an H attache. In reality the bond order is more complex But the nitrogen can accept an electron pair donating its formal pi bond to the oxygen that is formally double bonded to it.
Write Lewis structure of the following compounds and show formal charge on each atom. In the Lewis structures of N F 3 and C O 3 2 nitrogen and carbon occupy the central position whereas fluorine and oxygen occupy the terminal positions. Show how its stronger acid than HNO3.
In order to calculate the formal charges for HNO3 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding ele. In HNO 3 Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. Lewis structure of NO 3 1- after assigning the charge the net charge will be -1.
Home Draw the lewis dot structure of H2SO4 show formal charges. Anything that can accept an electron pair. If we draw it like the one on the right for hno3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds.
The structure of NO 3 1- is. The oxygen atoms are marked with letters to distinguish them from each other H-O-N-O. In this bond which of the following best describes.
13 The compound methylamine CH3NH2 contains a C-N bond. Of course the molecule is neutral and the Lewis structure reflects this. The hybridization of the atoms in this idealized lewis structure is given in the table below.
When we must choose among several Lewis structures with similar distributions of formal charges the structure with the negative formal charges on the more electronegative. Drawing the Lewis Structure for HNO 3. Which of the following are correct Lewis structures including formal charges for nitric acid HNO3.

Write Lewis Structure Of The Hno3 And Show Formal Charge On Each Atom

The Lewis Structure Of Hno3 Chemistry Stack Exchange
Solved Which Of The Following Are Acceptable Lewis Structures Including Formal Charges For Nitric Acid Hno3 Course Hero
Write Lewis Structure Of The Following Compounds And Show Formal Charge On Each Atom Hno3 No2 H2so4 Sarthaks Econnect Largest Online Education Community
Hno3 Nitric Acid Lewis Structure

How To Calculate The Formal Charges For Hno3 Nitric Acid Youtube

Draw A Lewis Structure For Nitric Acid The Hydrogen Atom Is Attached To One Of The Oxygen Atoms Brainly Com
Chemistry Net 05 01 2011 06 01 2011
Draw The Lewis Structure Of Nitric Acid Hno 3 Chegg Com

The Lewis Structure Of Hno3 Chemistry Stack Exchange
What Are The Lewis Structures For Hno3 Quora
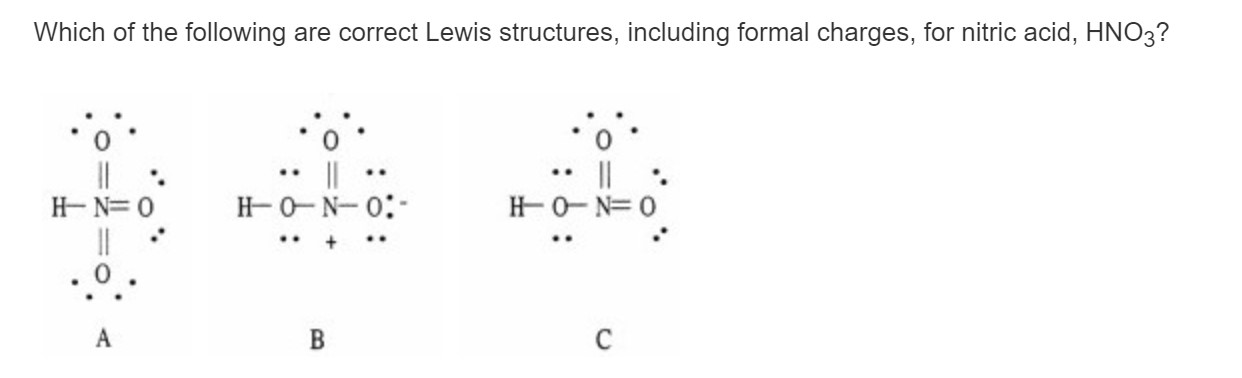
I I Know Why B Is Correct But Why Is C Wrong Chegg Com

How Is The Lewis Dot Structure For Nitric Acid Determined Quora

Consider The Lewis Structure For The Nitri Clutch Prep

1 Draw The Lewis Structure Of Nitric Acid Hno 3 That Minimizes Formal Charges Assign Lone Pairs Radical Electrons And Atomic Charges Where Appropriate 2 Calculate The Electrons Required Er Study Com
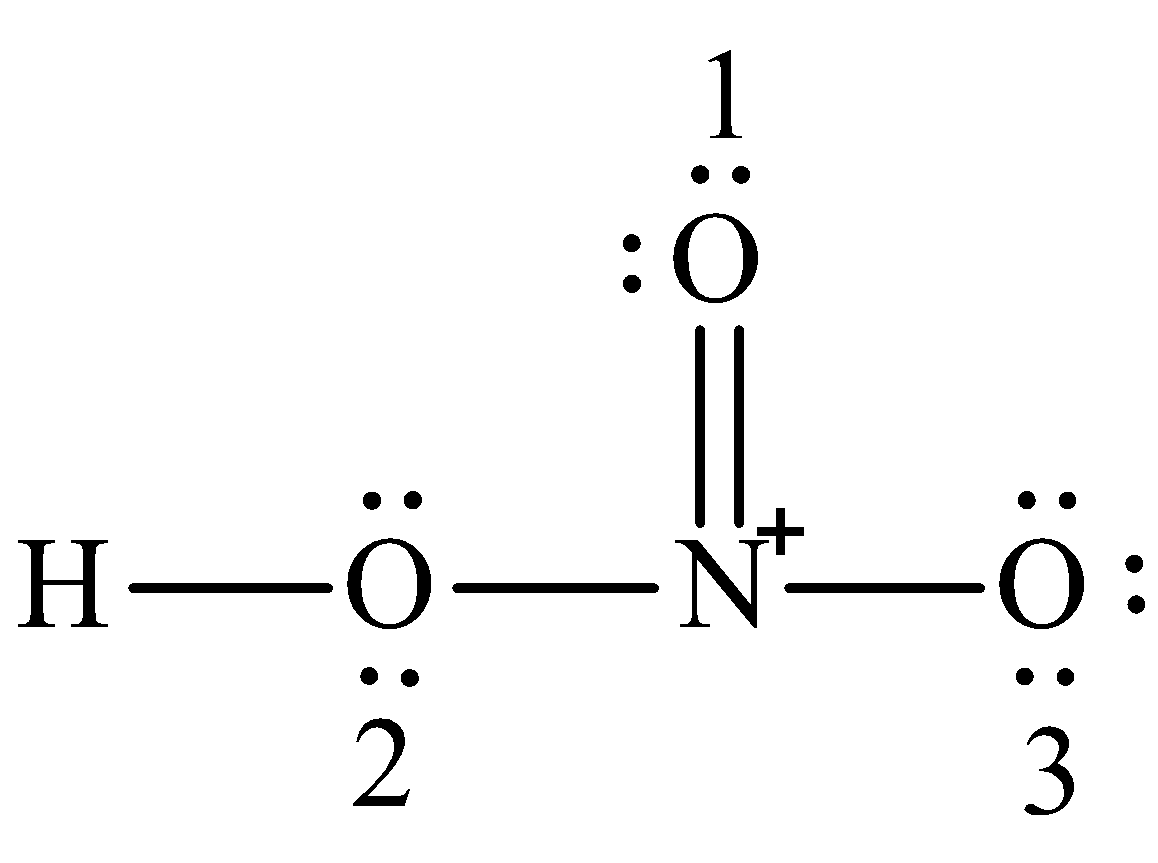
Write Lewis Structure Of The Hno3 And Show Formal Charge Class 12 Chemistry Cbse