N2h4 Lewis Structure Shape
N 2 H 4 is straightforward with no double or triple bonds. In this tutorial we will draw the lewis dot structure of AlCl3 with simple steps and all possible explanations.

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep
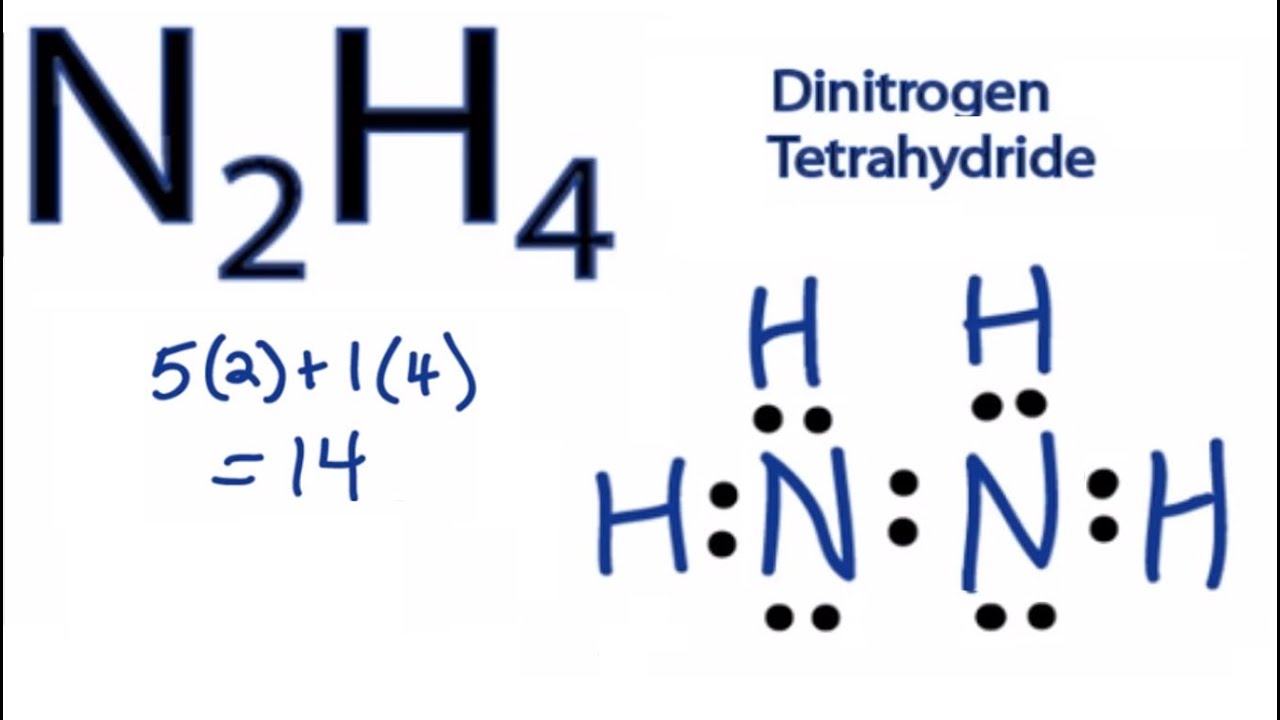
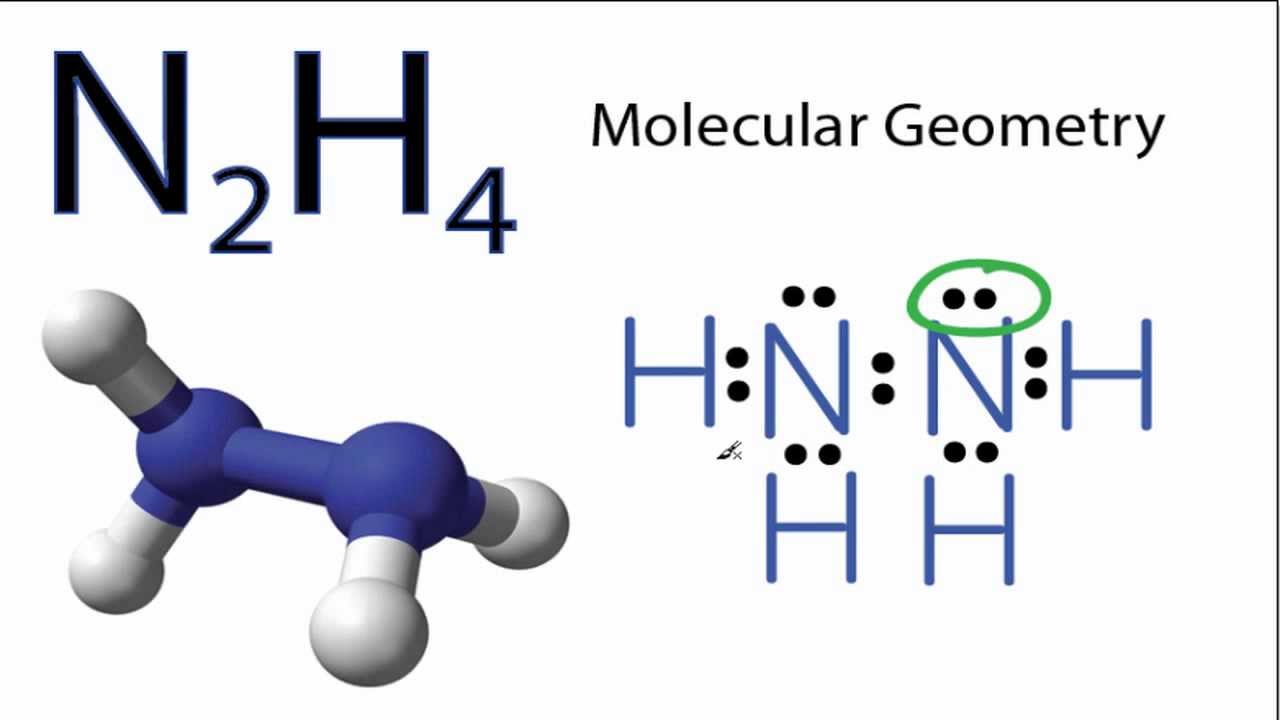
In the Lewis structure for N2H4 there are a total of 14 valence electrons.
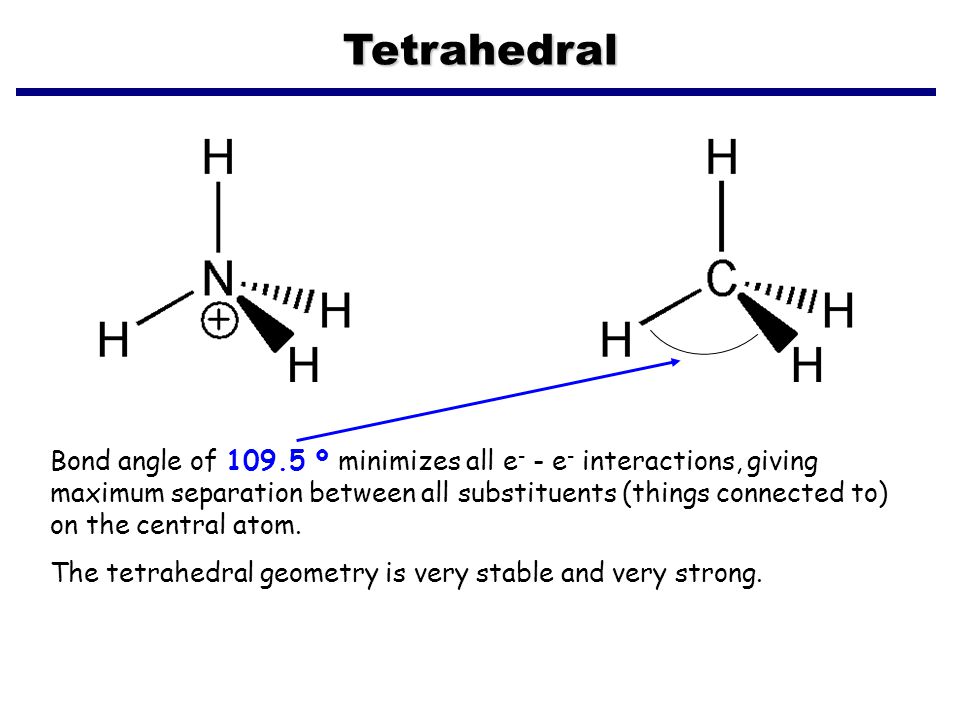
N2h4 lewis structure shape. CS2 form a cyclic molecule righta Draw Lewis structures for N2H4 and CS2b How do electron-group arrangement molecular shape and hybridization of N change when N2H4 reacts to form the productc How do electron-group arrangement molecular shape and hybridization of C change when CS2 reacts to. Determine the molecular geometry of N2H4 skeletal structure The molecular geometry is about each interior nitrogen atom of n2h4 is Trigonal pyramidal. It has four bonding pairs of electrons and two lone pairs of electrons.
Answered 1 year ago Author has 28K answers and 9815K answer views. N2H4 is straightforward with no double or triple bonds. The 3-dimensional geometrical structure of ammonium NH4 is referred to as Tetrahedral.
N2h4 Lewis Structure How To Draw The For Play Download. It can adapt two different geometrical forms cis and trans each with distinct symmetry. Each Nitrogen atom forms a single bond with one Hydrogen atom and a double bond with the neighboring Nitrogen atom.
For structure calculate total number valence elect. What is the Lewis structure for N2H4. Lewis structure of N 2 F 4.
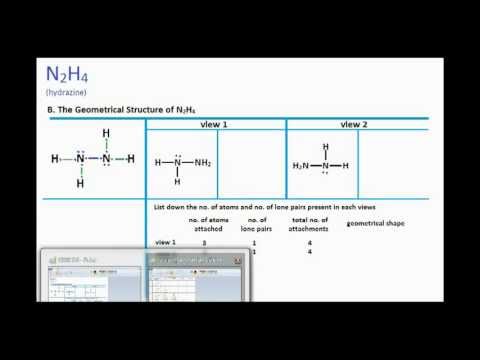
This tutorial will help you deal with the lewis structure and molecular geometry for hydrazine n2h4. Chemistry learning made easy. N2O4 consists of two central nitrogen atoms each bonded to two oxygen atoms.
Solutions for Chapter 11 Problem 43P. Hydrogen H only needs two valence electrons to have a full outer shell. Well determine the n2h4 molecular geometry with respect to nitrogen on right the other atom will have same shape since they are symmet.
Hydrogen H only needs two valence electrons to have a full outer shell. One can draw the 3-dimensional structure of an atom once they have the Lewis Structure of an atom. N2x510 F4x728 Total38 Step2.
Find octet e- for each atom and add them together. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
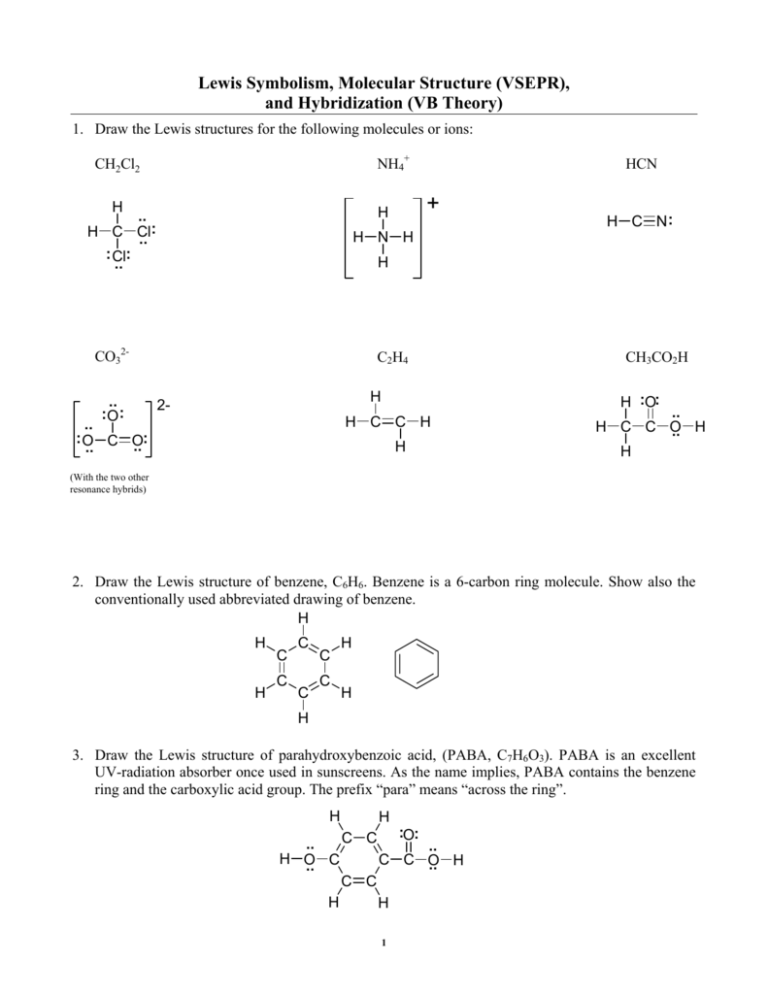
The molecule has a trigonal pyramidal shape with a bond angle less than 1095. Hydrazine N2H4 and carbon disulfide. The Lewis structure model combined with valence shell electron pair repulsion VSEPR can be used to predict many structural features of covalently bonded molecules and ions.
There are 12 valence electrons for this molecule. For total number v. The central atom of the AlCl3 lewis structure is exceptional to the octet rule as it holds stability with only 6 electrons.
In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Step method to draw lewis structure tetrafluorohydrazine. AlCl3 lewis structure contains three bonded pairs and a total of 9 lone pairs.
Metallic The bonding in metals is characterized by delocalization of valence electrons. Nitrogen having 5 valence shell electrons along with 4. Nh2nh2 Lewis Structure How To Draw The For Hydrazine Play Download.
Lets do the N2H4 Lewis structure. Hydrazine N2H4 and carbon disulfide CS2 form a cyclic molecule with the following Lewis structure. In order for each atom to have an octet of electrons without any exceeding the octet the Lewis structure would show a single bond between the nitrogens with each nitrogen having a single bond to one oxygen and a double bond to the other oxygen.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. How do shape and hybridization about C and N. Hydrogen H only needs two valence electrons to have a full outer shell.
In the Lewis structure for N 2 H 4. Find valence e- in all atoms. N2H4 is straightforward with no double or triple bonds.
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

Molecular Geometry Vsepr Ppt Video Online Download
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
N2h4 Lewis Structure And Molecular Geometry Youtube

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
Why Does N N Dimethylhydrazine Have Hydrazine When It Has N2h2 Not N2h4 Hydrazine Why Isn T It Called Dimethyldiimide Quora
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep

Vsepr For 4 Electron Clouds Video Vsepr Khan Academy

Hydrazine N2h4 And Carbon Disulfide Cs2 Clutch Prep

Draw The Lewis Structure For N2h4 Predict The Electron Geometry And Molecular Geometry And State Whether The Molecule Is Polar Or Nonpolar Study Com
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Lewis Structures And Molecular Structure

Hydrazine N2h4 And Carbon Disulfide Cs2 Form A Chegg Com

What Is The Lewis Structure For N2h4 Study Com

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist