Draw The Lewis Dot Structure For The Co2 Molecule
As we know that carbon has 4 valence electrons and hydrogen has 1 valence electron. A step-by-step explanation of how to draw the CO2 Lewis Dot Structure Carbon DioxideFor the CO2 structure use the periodic table to find the total number.
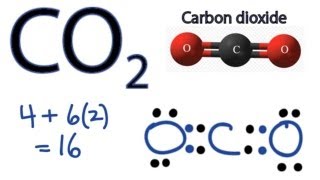
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule.

Draw the lewis dot structure for the co2 molecule. But we have two of them. The odd number immediately tells us that we have a free radical so we know that not every atom can have eight. What molecular geometry does carbon dioxide have.
I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. Your email address will not be published. On the periodic table Carbon is in group 4 or 14 sometimes.
Determine the electron geometry and molecular shape of this molecule. Determine the total number of valence outer shell electrons. You could alternatively also draw the structure by including two dots for every bond.
Lewis structure for c2h2 Lewis structure is a theory that helps in understanding the structure of a given. Carbon Dioxide CO2 8. Label the hydrogen and oxygen atoms to match the letter labels from the simulation A B and C.
CHEMISTRY What is the Lewis structure of c2h2o. A three-step approach for drawing the SCl4 Lewis structure can be used. Drawing CO2 Lewis Structure is very easy to by using the following method.
Draw the Lewis dot structure of CO2 molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. And then Oxygen is in group 6 or 16.
Were going to do the Lewis structure for CO2 Carbon dioxide. Key Points To Consider When Drawing The SCl4 Structure. People Also Asked What is the lewis dot structure for co2.
That would mean that you would have a total of eight dots around the carbon thereby filling its octet. For the AsO4 x molecule how many equal-energy resonance structures can you. Determine the electron geometry and molecular shape of this molecule.
How many sigma and pi bonds does it contain. Carbon is the least electronegative that means it stays at the center. Draw the Lewis structure for each organic compound from its condensed structural formulaa.
That means its going to go at the center. Make sure to WRITE the Electron Structure and Molecular Shape Names as well as their. Which atom is more electronegative carbon or oxygen answer below.
How to draw single bonds using dots to. So lets multiply that together there. So well put the Carbon right here.
So put the Carbon in the middle and then set the oxygen either side of that. Draw the Lewis dot structure for CO2. A step-by-step explanation of how to draw the COCarbon Monoxide Lewis Dot DiagramFor the CO structure use the periodic table to find the total number of v.
The first step is to sketch the Lewis structure of the SCl4 molecule to add valence electron around the sulfur atom. The second step is to valence electron to the four chlorine atoms and the final step is to combine the step1 and step2 to get the SCl4 Lewis Structure. The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule.
Draw the Lewis Dot Structure. Draw the lewis structure for the molecule c3h4. Lets draw the structure.
Show all bonding valenceelectron pairs as lines and all nonbonding valence electron pairs as dotsa. Draw the Lewis dot structure for CO32-. The sum of the valence electrons is 5 from N 6 from O 11.
Here in this post we described step by. Leave a Reply Cancel reply. According to Lewis-dot structure there are 16 number of bonding electrons and 0 number of non-bonding electrons.
Write a Lewis structure for each of the following simple molecules. The octets of both of the oxygen atoms are also satisfied since the oxygens have a total of eight electrons around them thereby filling the valence shell. Set the atom electronegativities of atom A B and C accordingly.
Is this molecule polar or. I also go over hybridization shape and bond angles. So total valence electrons are 16.
Draw the Lewis dot structure for carbon dioxide below. So we have 12 plus 4 16 total valence electrons. Keep in mind that in reality electrons are constantly moving around the nucleus.
Carbon is the least electronegative. Lewis structure for C2H2O. Are there bond dipoles in carbon.
To know the lewis structure of CO2 one should first understand what precisely the Lewis structure is. Also know how many total valence electrons are used in drawing the Lewis structure of carbon dioxide. Draw the Lewis Dot Structure.
By signing up youll get. The carbon dioxide chemical formula is CO2. This is the Lewis Dot Structure for CO2.
These valence electrons are represented by drawing dots around the individual atoms hence the Lewis dot structure. Total valence electron available for drawing the CO2 lewis dot structure 4 26 16 valence electrons. CO2 Lewis structure So CO2 4 62 16.
To draw the Lewis structure for an odd-electron molecule like NO we follow the same six steps we would for other molecules but with a few minor changes. Draw Lewis structures to figure this one out a 2 b 0 c -1 d -2 e -3. Make sure to WRITE the Electron Structure and Molecular Shape Names as well as their bond angles.
Drawing lines represent the bonds formed in the molecule.

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Lewis Structure Practice Worksheet 4 Stepsa Ch4 Lewis Structure In 2020 Practices Worksheets Graphing Linear Equations Chemistry Worksheets

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Covalent Molecules Gif 546 404 Teaching Chemistry Carbon Molecule Scientist Birthday Party

Linear Molecular Geometry Molecular Geometry Chemistry Projects Teaching Biology

So2 Lewis Structure How To Draw Lewis Dot Structure For So2 Sulfur Dioxide Electron Configuration Chemistry Worksheets Lewis

Co2 Lewis Structure Carbon Dioxide Youtube
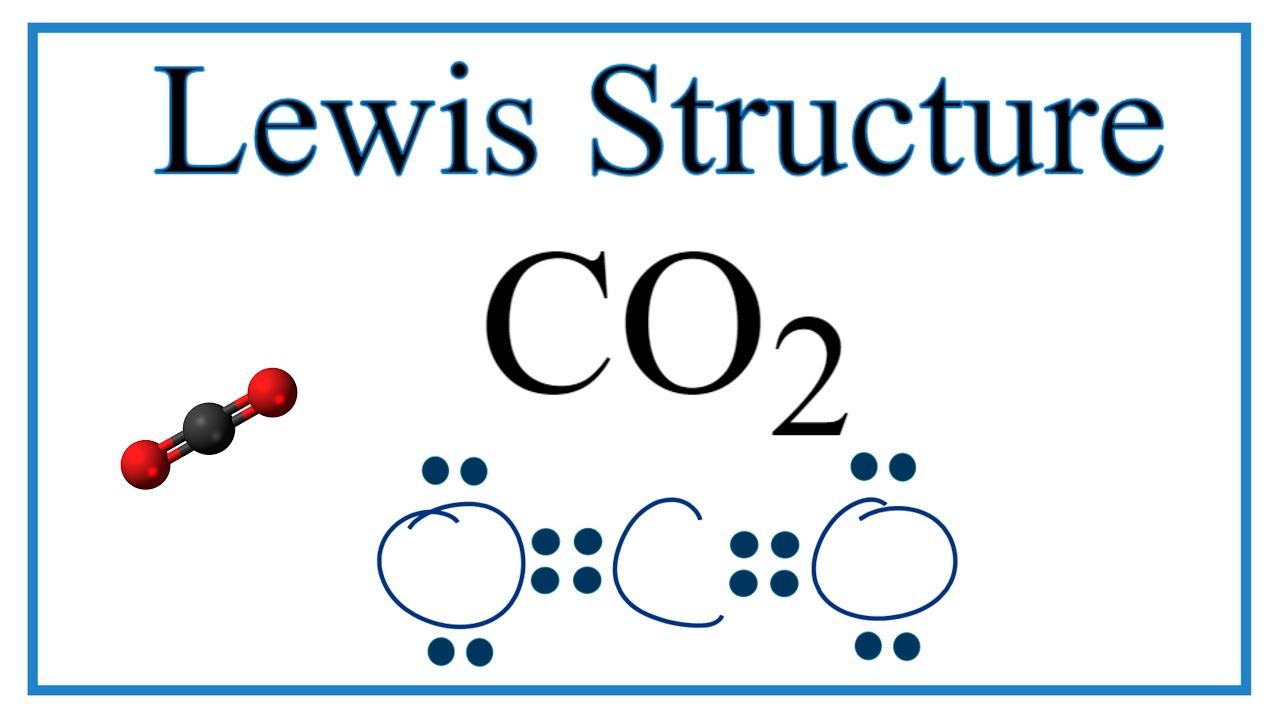
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube
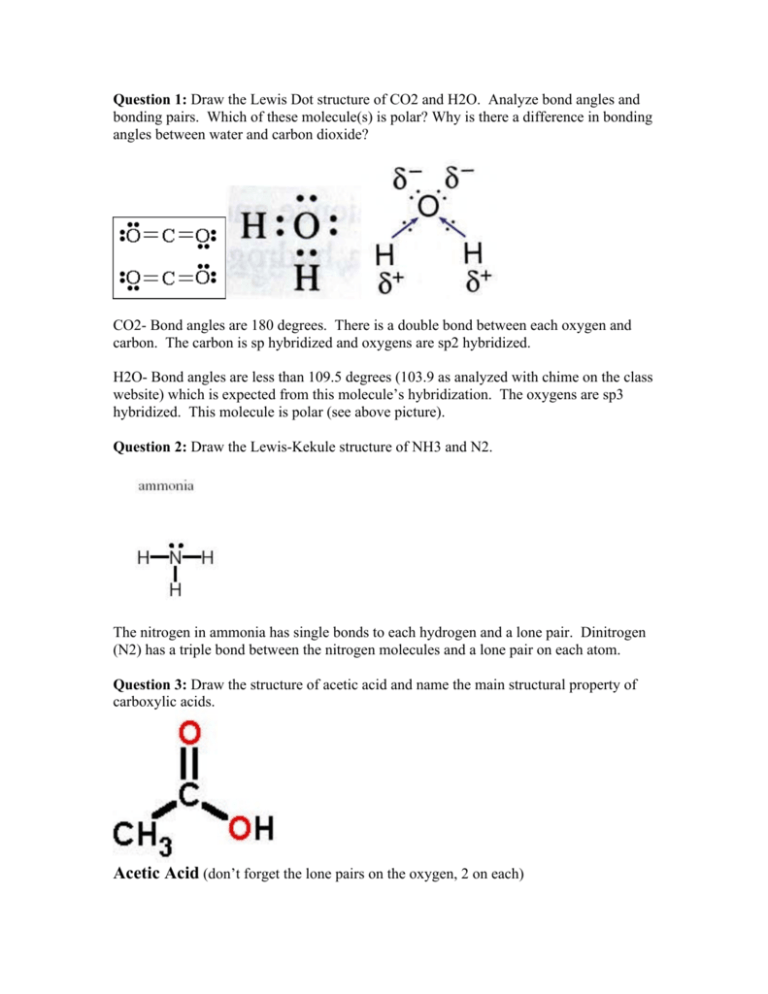
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze
Co2 Lewis Structure Molecular Geometry And Hybridization

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Co2 Lewis Structure Molecular Geometry And Hybridization Molecular Geometry Molecular Lewis
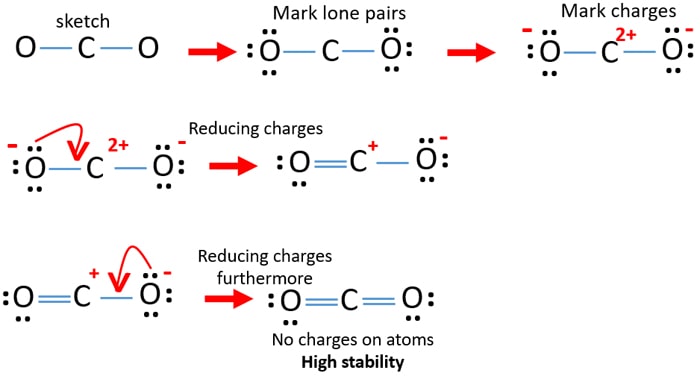
Co2 Carbon Dioxide Lewis Structure And Shape

Lewis Structure For Co Carbon Monoxide Youtube

The Structure Of A Carbon Dioxide Molecule Covalent Bonding Carbon Dioxide Carbon

Co2 Lewis Structure Carbon Dioxide Youtube

Lewis Dot Structure For Hydrogen H Lewi Electron Configuration Chemistry Worksheets Chemical Equation

In Chemistry Drawing Lewis Dot Structures Can Be Challenging But They Provide A Wealth Of Information About The Molecules Th Chemistry In Pictures

Co2 Lewis Structure Easy Hard Science