Ethylene Glycol Lewis Dot Structure
Ethylene Glycol based water solutions are common in heat-transfer applications where the temperature in the heat transfer fluid can be below 32 o F 0 o CEthylene glycol is also commonly used in heating applications that temporarily may not be operated cold in surroundings with freezing conditions - such as cars and machines with water cooled engines. Ethylene glycol is similar in structure to glycerol.
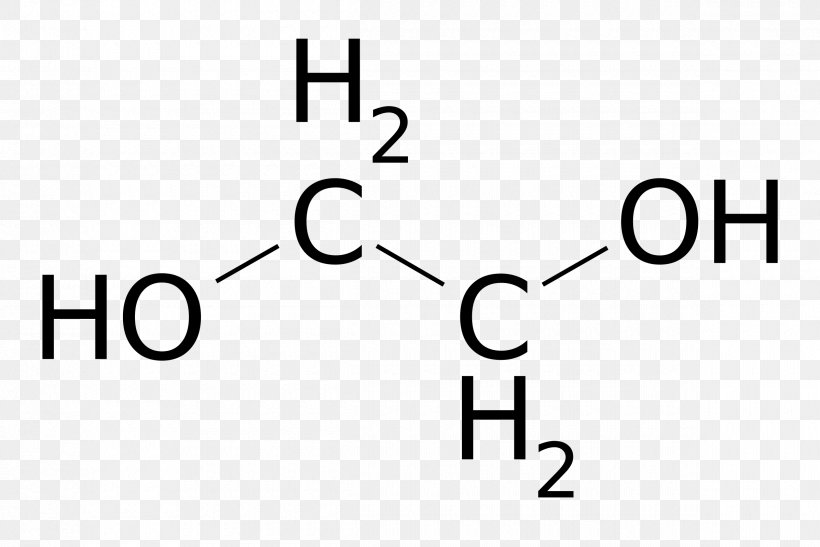
Ethylene Glycol Structural Formula Molecule Ethylene Oxide Png 2400x1602px Ethylene Glycol Area Black Brand Chemical Compound
Question A voltaic cell consists of a half-cell and a half-cell at 25.
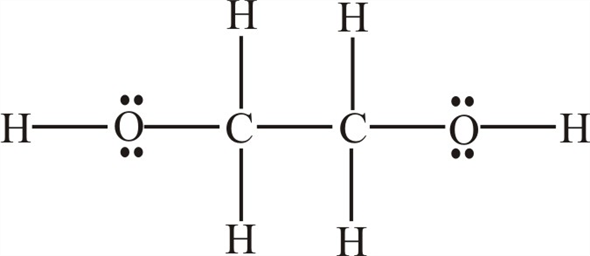
Ethylene glycol lewis dot structure. 18 bonding and 4 non-bonding electrons B. Although ethylene glycol has two alcohol groups its main application is as the main ingredient in antifreeze and not for fuel purposes. B Draw the Lewis dot structure of chloroethane C 2 H 5 Cl.
C Chloroethane has a slightly higher molar mass than ethylene glycol but a much lower boiling point 3. The initial concentrations of and are 140 and 0100. In the molecule ethene both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital.
Ethylene glycol lewis dot structure oh chapter draw each problem. Chloroethane-11-d2 C2H5Cl CID 16217388 structure chemical names physical and chemical. Get college assignment help at Smashing Essays Question What is the lewis dot structure for SiF2.
I could put in lone pairs of electrons on the oxygen or I could leave them off. 107-21-1 Revision Date 2007 -07 -01 SARA 311312 Hazards Acute Health Hazard Chronic Health Hazard Massachusetts Right To Know Components Ethylene glycol CAS-No. Question A voltaic cell consists of a half-cell and a half-cell.
Monochloroethane 75-00-3 101-27-9 107-14-2 624-65-7. Ad_1 Get college assignment help at Students Paper Help Question What is the lewis dot structure for SiF2 Question A voltaic cell consists of a half-cell and a half-cell Question A voltaic cell consists of a half-cell and a half-cell at 25. It is odorless but has a sweet taste.
A Draw the Lewis dot structure of ethylene glycol. A Draw the lewis dot structure of ethylene glycol. Ethylene glycol 107-21-1 HO-CH2-CH2-OH.
16 bonding and 4 non-bonding electrons E. The initial concentrations of and are 140 and 0100 respectively. Draw the Lewis dot diagram for the ethylene glycol molecule A.
These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped probability areas above and below the plane of the six atoms. B Draw the Lewis dot structure of chloromethane C2H5cl c Chloroethane has a slightly higher molar mass than ethylene glycol but much lower. The correct Lewis structure for ethene is shown below.
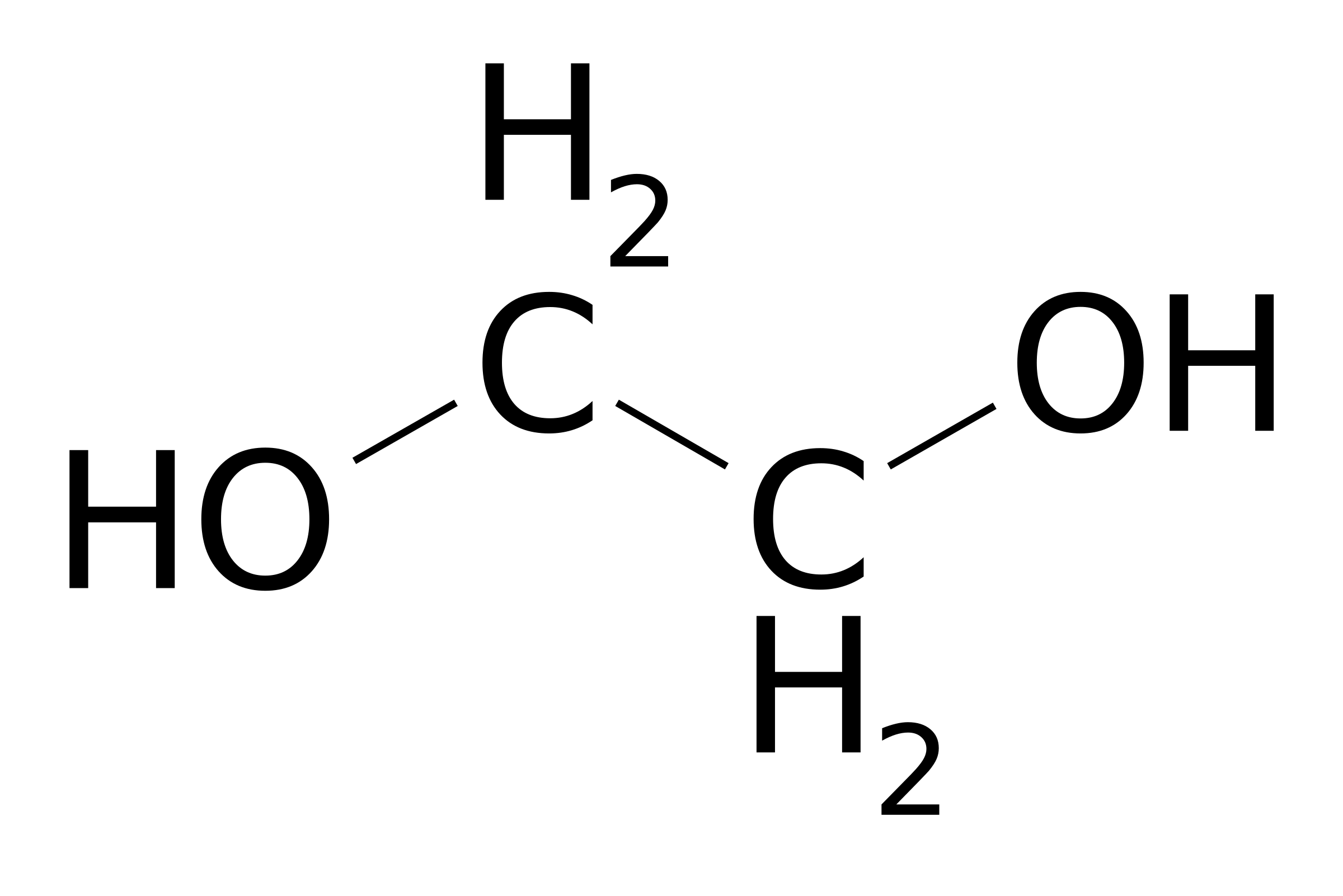
Ethylene Glycol Formula Structure. In the Lewis structure of ethylene glycol HOCH2CH2OH how many electrons are bonding and how many are non- bonding. A Draw the lewis dot structure of ethylene glycol.
A Draw the lewis dot structure of ethylene glycol. This reaction can be catalyzed by either acids or bases or can occur at neutral pH under elevated temperatures. 107-21-1 Revision Date 2007-07-01.
The condensed structural formula is a system of writing organic molecules in shorthand notation with more details that the molecular formula but lesser extended that the normal structural formula. 107-21-1 Revision Date 2007-07-01 Pennsylvania Right To Know Components Ethylene glycol CAS-No. Glycerol is a C3 triol and ethylene glycol is also a polyol but a C2 diol.
22 bonding and 0 non-bonding electrons C. Structure properties spectra suppliers and links for. Ethylene glycol is a synthetic liquid substance that absorbs water.
Density massvolume Mass of H number of moles relative molecular mass 1 1 1 g density 1624x1015 8065x10-16gL 8116 Ethylene glycol C2H6O2 has one OH bonded to each carbon. CH 3 CH 2 ClCHClCH 2 Cl. Ethylene glycol is produced from ethylene ethene via the intermediate ethylene oxideEthylene oxide reacts with water to produce ethylene glycol according to the chemical equation.
Ethylene glycol is used to make antifreeze and de-icing solutions for cars airplanes and boats. Ethylene glycol C 2 H 6 O 2 has one OH bonded to each carbon. C 2 H 4 O H 2 O HOCH 2 CH 2 OH.
It is also used in hydraulic brake fluids and inks used in stamp pads ballpoint pens and print shops. Lewis Dot Structure Of Ethene Alkeenit Wikipedia 화합물 사전 에테인Ethane 인포라드infoRAD Corp Write the molecular formula of the following compounds and Chemistry X Carbon and its Compounds Saturated and. Brief chemistry video describing ethylene glycol and one area its used.
18 bonding and 8 non-bonding electrons D. In the Lewis structure for NOF there are a total of 18 valence electrons.

Why Does Ethylene Have A Double Bond And Ethylene Glycol Does Not Have A Double Bond Quora
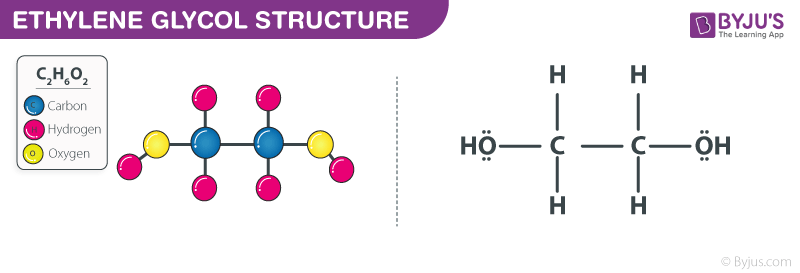
What Is Ethylene Glycol C2h6o2 Formula Structure Properties Uses
Ethylene Glycol C2h6o2 Chemspider
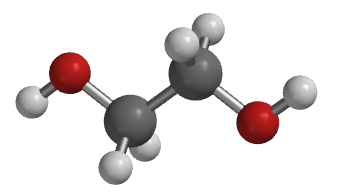
Illustrated Glossary Of Organic Chemistry Ethylene Glycol
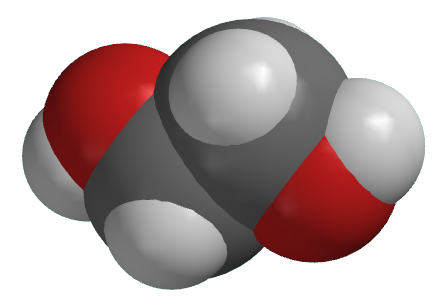
Illustrated Glossary Of Organic Chemistry Ethylene Glycol
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Ethylene Glycol Molecule Of The Month June 2018 Html Version

Ethylene Glycol Properties Uses Structure Britannica
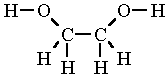
Ethylene Glycol Formula Structure
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Solved Chapter 8 Problem 116ap Solution Masteringchemistry Standalone Access Card For Fundamentals Of General Organic And Biological Chemistry 7th Edition Chegg Com
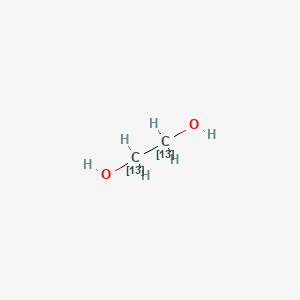
Ethylene Glycol 13c2 C2h6o2 Pubchem

Is Ethylene Glycol Molecular Or Ionic Socratic
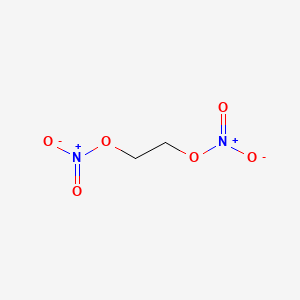
Ethylene Glycol Dinitrate C2h4n2o6 Pubchem
Ethylene Glycol Molecule Of The Month June 2018 Html Version

What Is Ethylene Glycol C2h6o2 Formula Structure Properties Uses
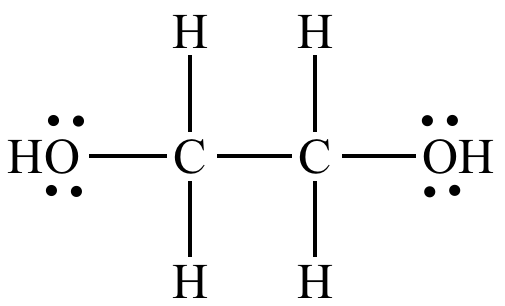
Illustrated Glossary Of Organic Chemistry Ethylene Glycol
Ethylene Glycol Molecule Of The Month June 2018 Html Version