H2so4 Lewis Structure Formal Charge
There are no lone pairs on nitrogen atom and also there are charges on one oxygen atom and nitrogen atom. Je comprends pas un truc sur la formule de lewis de h2so4.

Formal Charge And Dot Structures Chemical Bonds Chemistry Khan Academy Chemical Bond Chemistry Organic Chemistry
Formal charge valence electrons - non bonding valence - bonding valence2.

H2so4 lewis structure formal charge. Formula-3 Formal ChargeValence Electrons-Dots-Lines The formal charge of each hydrogen atom in CH2CH2 is zero and the formal charge of each carbon atom is also zero. This structure of H 2 SO 4 is more stable than previous structures. This the lewis dot structure of h2so4.
There is a NO bond in nitric acid lewis structure. While PM7 isnt a highly accurate method the difference in stability isnt close. Now we will find O O 6 4 42 0 O 0.
In the lewis structure for h 2 so 4 there are a total of 32 valence electrons. Also note that you should put the HSO4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. We will see how to make it better in step 8.
Draw The Lewis Structure For H2so4. So the entire so3 molecules has zero formal charge. What is the molecular geometry of the molecule.
Click hereto get an answer to your question Write Lewis structure of the H2SO4 and show formal charge on each atom. Each cl atom interacts with eight valence electrons total. Now there are no charges on atoms.
Now first we will find formal charge of sulfur S S 6 0 122 0 S 0. To find the best Lewis structure we have to find out the formal charge of each atom in CH2CH2 by applying the formula for formal charge. Therefore 2 valence for H of valence for S 4 valence for O.
Draw The Lewis Structure For H2so4 lewis structure draw lewis structures acid sulfuric electrons valence structure resonance draw octet rule many bonded shapes each brainly lewis structure drawing begingroup stack lewis acid sulfuric draw structures bonded. H2so4 lewis structure molecular geometry. So when you see something like Sulfur there make sure you check the formal charges.
So this structure has more chance to be the lewis structure of H 2 SO 4. Chage on H is 1 on O is -2 on sulphur we need to find let us take it as x Charge as whole on h2so4 is 0 21x4-20. The lowest energy geometry is the traditional H X 2 S O X 4 Lewis structure estimated at Δ H f 0 -17788 kcalmol.
We could go on expanding the s octet but this would start to move the formal charges away from zero. Formal charge on an atom in a Lewis structure total number of valence electrons in free atom total number of non-bonding lone pairs electrons 12 total number of bonding or shared electrons Answer verified by Toppr Upvote 0 Was this answer helpful. Formal charge is also called oxidation no.
Notice that the sum of the absolute values of the formal charges is 4 although this species is uncharged. Formal charge valence electrone non bonding valence electrone bonding electrone2. That makes this a much better structure for the sufuric acid molecule.
Lewis structure of nitric acid. 2 ikatan kovalen tunggal c. Draw the Lewis dot structure and calculate the formal charges of the oxygen and sulphur atoms of H2SO4.
In new structure charges of atoms are reduced than previous structure. In HSO 4-Lewis structure Sulfur is least electron electronegative atom and goes in the center of the Lewis structure. 70 more lewis dot structures.
So the formal charge of Oxygen is 0. In the Lewis structure. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
There are two lewis structures for. When we form those two double bonds and recalculate our formal charges well find that the formal charge on each atom in the H2SO4 Lewis structure is now 0. In order to calculate the formal charges for H2SO4 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding el.
The formal charges in a structure tell us the quality of the dot structure. For the Lewis structure for HSO 4-you should take formal charges into account to find the best Lewis structure for the molecule. So the formal charge of sulfur is 0.
You can see those signs in the following figure. Structure lewis dot draw bond chemistry oxygen class acid charge stable formal.

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots

H2so4 Lewis Structure Sulfuric Acid Youtube

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
Can You Calculate The Formal Charge On Each Oxygen In Sulphuric Acid Quora

Write Lewis Structure Of The Following Compounds And Show Format Charge On Each Atom Hno 3 No 2 H 2 So 4

How To Calculate The Formal Charges For H2so4 Sulfuric Acid Youtube
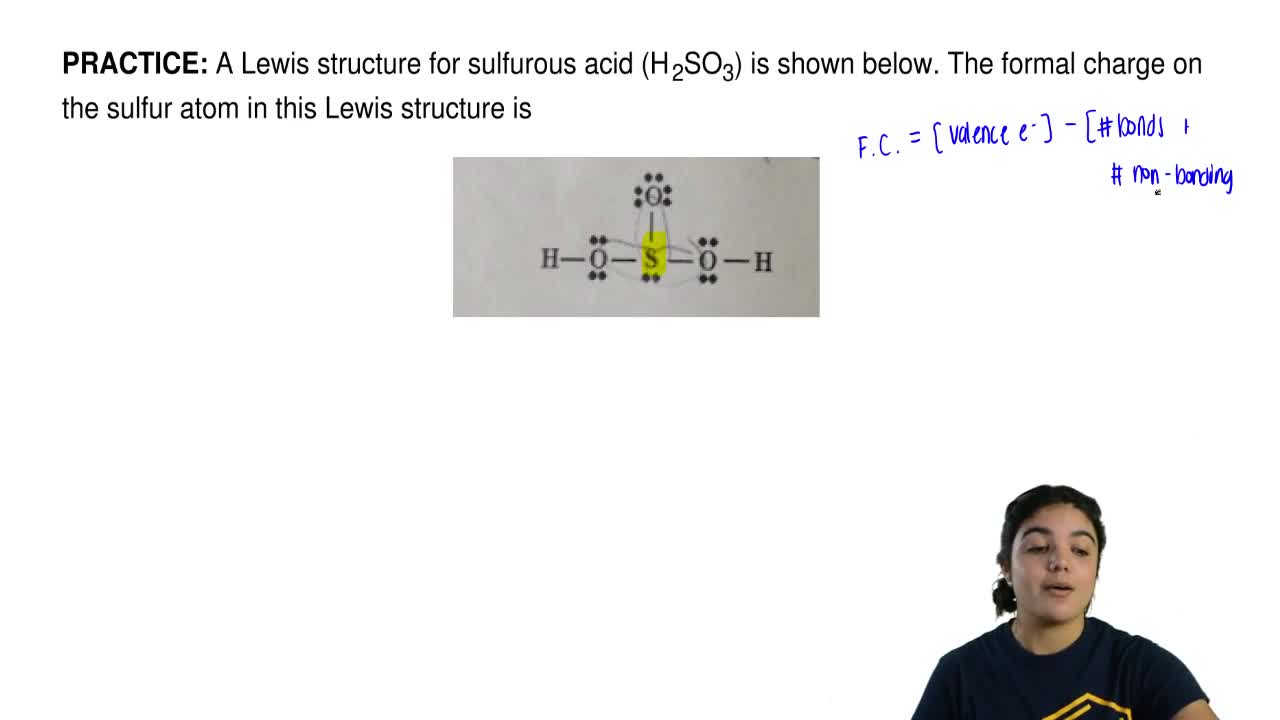
A Lewis Structure For Sulfurous Acid H 2so3 Is Shown Below The Formal Charge On The

Write Lewis Structure Of The Following Compounds And Show Format Charge On Each Atom Youtube

Write Lewis Structure Of The Following Compounds And Show Formal C

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots
H2so4 Lewis Structure How To Draw The Lewis Structure For H2so4 دیدئو Dideo
Lewis Dot Structure And Explanation Of H2so4 And Co Chemistry Topperlearning Com Dpr163cc
Can You Calculate The Formal Charge On Each Oxygen In Sulphuric Acid Quora

Hapticity Electron Contribution Formal Charge And Oxidation State Calc Oxidation State Oxidation Electrons

Non Aqueous Solvents Nh3 Hf H2so4 N2o4 Pocl3 Socl2 Brf3 In 2021 Solvents Chemistry Agno

O3 Lewis Structure How To Draw The Dot Structure For O3 Youtube