How Many Valence Electrons Does Pcl3 Have
Once we know how many valence electrons there are in PCl5 we can distribute them around the central atom and attempt to fill the outer shells of each atom. Since it is in Group 5 it will have 5 valence electrons.

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium
How many ELECTRON PAIRS does PCL3 has.

How many valence electrons does pcl3 have. Total number of valence electrons of PCl3. CH 4 NH 3 H 2O CCl 2F 2 SOCl 2 4 SO 4 2-zWorked Ex. How Many Valence Electrons Does PCl3 HaveWhat is the number of valence electrons in PCl3How many valence electrons are in a phosphorus trichloride PCl3.
B Draw a valid lewis electron dot Structure for carbonate ion CO 3 2- accounting for all valence electrons. How many lone pairs does this molecule have. So you should count over and find that P has 5 O has 6 and Cl has 7 valence electrons.
Phosphorus 15 protons 16 neutrons and 15 electrons Level 1 2 electrons Level 2 8 elec Level 3 5 elec. Thus phosphorus has five valence electrons. Each Cl atom has 7 valence electrons so the Lewis structure for PC13 will include 21 electrons.
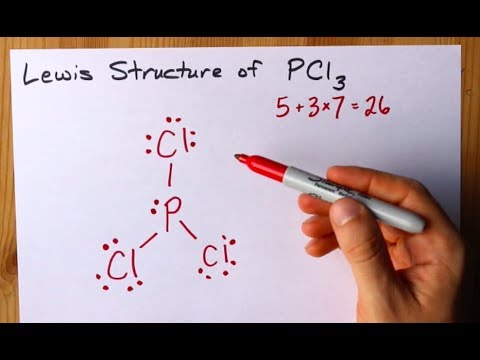
Phosphorus Trichloride PCl3 has a total of 26 valence electrons. In the PCl 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. Each Chlorine forms single bond with phosphorus to complete the octet This leaves it with 2 bonded and 6 non-bonded electrons These 6 non-bonded electrons are present in the form of lone pairs.
When do you have to. In the Lewis structure for PCl 3 there are a total of 26 valence electrons. How many electrons are in PCl3.
What happens when bond electrons. How many VALENCE PAIRS does PCl3 has. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
EXAM 2 BUM BUM BUMMM. Whereas in PCl5 there will be two electrons left over which is why it is an exception to the octet rule. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of five electrons present in the valence shell of phosphorus 3s²3p³.
3 for each Cl makes it 9lone pairs for chlorine. You lose 1 electron from each element and theres 2 electrons per bond. How many valence electrons does pocl3 have.
How many BONDING PAIRS does PCl3 has. Neuro exam 2 practice questions. SF 6 BrF 5 or when you simply have too many electrons eg.
Phosphorus Trichloride PCl3 has a total of 26. Generally do not give anything an expanded octet unless you have to. 5 73.
1 See answer oli3ndamari is waiting for your help. 3 Electron-Dot Structures 1 How many total valence electrons. Solution for How many electrons does phosphorus have in its.
After the bonds have been formed in PCl 3 how many valence electrons remain. Phosphorus has 5 valence electrons. We review their content and use your feedback to keep the quality high.
What is the total number of valence ELECTRONS. Experts are tested by Chegg as specialists in their subject area. 2 Does every atom have an octet.
When you have more than 4 atoms attached to a central atom eg. - 2273502 oli3ndamari oli3ndamari 11222016 Physics High School answered How many valence electrons does pocl3 have. How many VALENCE PAIRS does PCl3 has.
Draw or state the shape of. A Draw a valid lewis electron dot Structure for phosphorus trichloride PCl 3 accounting for all valence electrons. PCl3 has 10 lone pairs Each Chlorine atom has 7 initial valence electrons.
XeO 2 BrF 3. In PCl3 Ps three valence electrons are evenly distributed among the three chlorine atoms. Valence electrons of Phosphorus Valence electrons of Chlorine.
Problems 74 75 Multiple Bonds. Draw the Lewis Dot Structure for O3. How many electrons are in PCl3.
PCl3 is a STABLE molecule because all the atoms of the molecule has 8 valence electrons shared Is pcl5 a Lewis acid. OTHER SETS BY THIS CREATOR. Add your answer and earn points.
Hree pairs will be used in the chemical bonds between the P and Cl. According to the periodic table above phosphorus belongs to Group 5A. ZHl bdhlH only gets one bond.
100 3 ratings Previous question Next question. ZDraw structures for the following. Draw or state the shape of PCl 3 molecules.
What is the total number of valence ELECTRONS. Halogens usually only form single bonds. Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3.
Who are the experts.
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Bf4 Lewis Structure How To Draw The Lewis Structure For Bf4 Youtube

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Drawings Dots Lewis

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Hybridization Of Of2 Oxygen Difluoride In 2021 Molecules Oxygen Things To Come

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

How Is The Electron Dot Structure Of Pcl3 Determined Quora

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons