How To Determine Double Bonds In Lewis Structures
Now you need to determine the FORMAL CHARGES for all of the atoms. See the figure below.

O2 Lewis Structure Easy Hard Science
Count the valence electrons in your trial structure 20.
How to determine double bonds in lewis structures. Ethyne C 2 H 2 is a linear molecule with a triple bond between the two carbon atoms see Figure 4. Try to satisfy the octets of the atoms bydistributing the remaining valence electrons as nonbondingelectrons. For this example SO2 and SO3 have double bonds you can discover this through formal charge and SO3 2- has one double bond and two single bonds.
Distribute the remaining electrons to the central atom as non-bonding pairs Form double and triple bonds until the central atom has a full octet. This gives an idea about the chemical bonding number of lone pairs the number of single bonds or double bonds number. Write the skeleton structure of the molecule.
In the Lewis Structure electrons are depicted as dots. Draw nonbonding pairs around the outer atoms until they have a full octet. 1 N 2 O 1 17 26 1 18.
Ensure that all of. The bond order will be equal to 4 3. This is the total number of electrons that must be used in the Lewis structure.
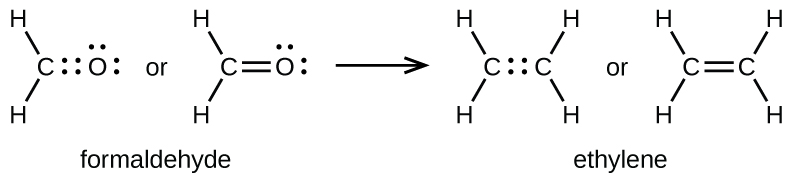
In the last video we saw how to draw dot structures for molecules with single covalent bonds and this video will talk about multiple covalent bonds and so we start the same way we did in last video if I want to draw the dot structure for c2h4 I would find carbon over here and once again carbon is in Group four so it has four valence electrons so Im going to go ahead and put in one carbon with. General rules for drawing Lewis structure Count up the total number of valence electrons. Formal Charge V - L B2.
The formal charge is calculated by. This is the correct Lewis structure for carbon dioxide which has two double bonds. Group number of atom - ½ number of bonding electrons - number of lone pair electrons ie.
In a conventional Lewis electron-dot structure a double bond is shown as a double dash between the atoms as in CC. When figuring out whether to place a double or triple bond you should always look at the number of valence electrons present as well as the number of bonds a central atom is likely to form. Now each atom is surrounded by eight electrons and the total number of electrons is sixteen as required.
Use two valence electrons to form each bond inthe skeleton structure. The trial structure has two more electrons than are available. Calculate the total number of electrons that would be needed for each atom to have an octet or doublet for H.
A bond between two electrons is represented by a line marked by a dot at both ends involving the participating electrons. One is a sigma bond while the other is a pi bond. Now count the valence electrons you actually have available.
Another good way to know whether to use double or single bonds is to calculate the formal charge on each atom in the molecule. Draw a new trial structure this time inserting one double bond for each extra pair of electrons. The whole procedure for writing Lewis structures can be explained but to first answer your question always start with single bonds then make double bonds if its required.
Subtract the result of step 1 from the result of step 2. Determine the total number of valenceelectrons. Firstly draw lewis structure then count the total number of bonds which is equal to 4 here.
So heres a quick and flexible procedure. To find these you draw Lewis structures and count formal charges. For a molecule we add the number of valence electrons use the main group number on each atom in the molecule.
A double bond involves two atoms shar- ing two pairs of electrons. A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. Add up he total number of valence electrons for.
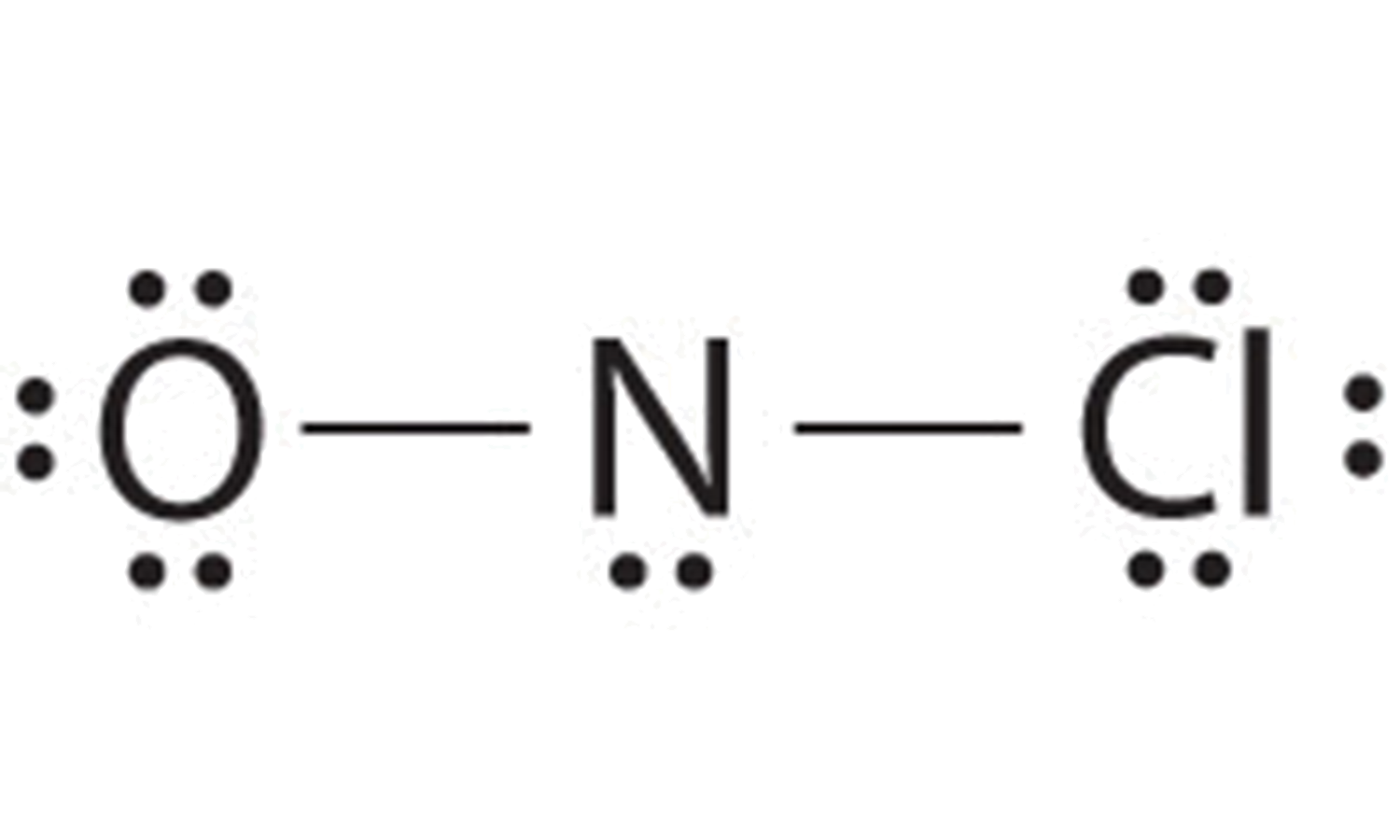
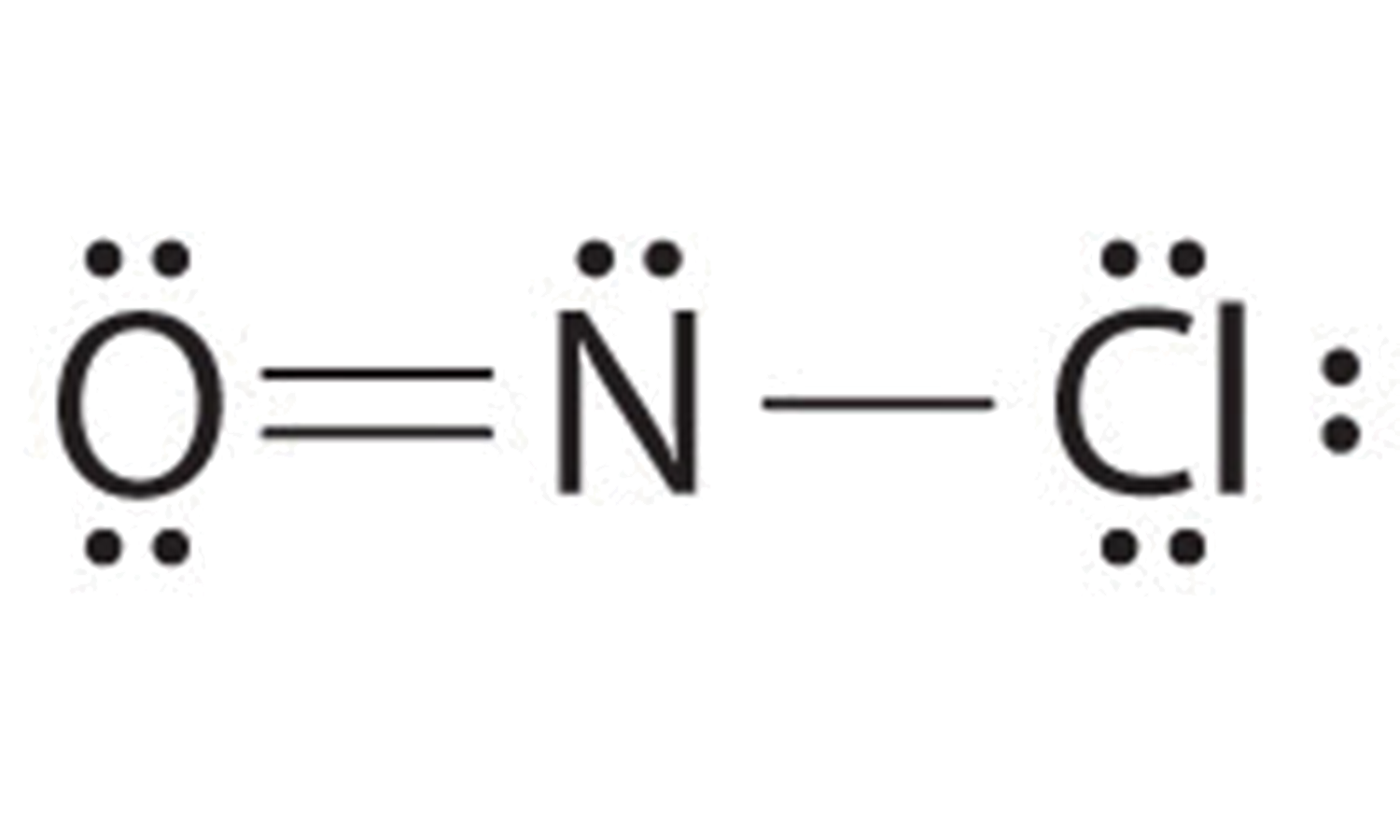
Finally count the number of bond groups between individual atoms which is 3. O-NO and ON-O two possibilities Right here the two possibilities tell us that that we. Determine the total number of valence outer shell electrons in the molecule or ion.
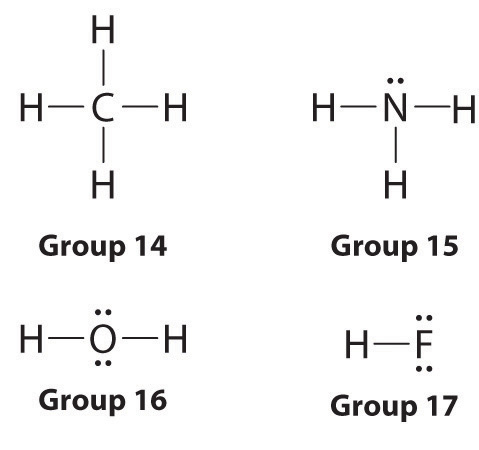
It is important to realize however that the two bonds are different. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as Cl2 O2 OF2 CH4 NH3 H2O C2H2 and N2H4. Lewis Structure is otherwise called electron-dot structure.
A single bond involves two atoms sharing one electron pair. In this case the N is short 2 electrons so we can use a lone pair from the left most O atom to form a double bond and complete the octet on the N atom.

9 5 Covalent Bonding Lewis Structure Chemistry Libretexts

Multiple Bonds Chemistry For Majors
In A Covalent Bond S Lewis Dot Structure How Do I Know When To Use Double Bonds Or Single Bonds Quora

In A Covalent Bond S Lewis Dot Structure How Do I Know When To Use Double Bonds Or Single Bonds Quora

Types Of Bonds And Orbitals Notation Orbital And Lewis Dot Shmoop
9 5 Covalent Bonding Lewis Structure Chemistry Libretexts
4 2 Lewis Structures Problems Chemistry Libretexts

Lewis Structures In Organic Chemistry Chemistry Steps
7 3 Lewis Symbols And Structures Chemistry
How To Recognize Invalid Or Unlikely Lewis Structures

Double And Triple Covalent Bonds Introduction To Chemistry
Single And Multiple Covalent Bonds Article Khan Academy

Lewis Dot Structures For Covalent Compounds Part 1 Clear Simple Youtube
9 5 Covalent Bonding Lewis Structure Chemistry Libretexts
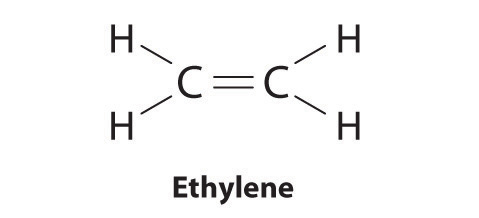
10 4 Writing Lewis Structures Chemistry Libretexts

Lewis Structures In Organic Chemistry Chemistry Steps
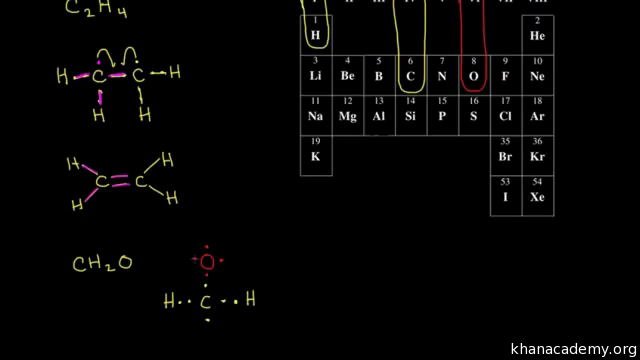
Dot Structures Ii Multiple Bonds Video Khan Academy


