Is Pf5 A Lewis Acid Or Base
The Lewis acid - base concept is a definition of acid and base which is independent of protons. It acts as a mild Lewis acid.
In order to obtain a Kr configuration it can add up to ten more electrons.
Is pf5 a lewis acid or base. None of the above is a Lewis acid. The bisulfite ion is amphiprotic and can act as an electron donor or acceptor. Because HF is a weak acid fluoride salts behave as bases in aqueous solution.
For example PF5 F- Pf6 is a lewis acid-base reaction. S b C l X 3 has a completely filled spd orbitals so it can no longer accept electrons hence doesnt act as a Lewis acid. A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct.
Which compound will be a stronger Lewis acid PF5 or PCl5Explain briefly. Fastest Reliable Cheapest Game Keys and Digital Services at DamnModz. What is the adduct that isformed Include a picture if you can.
A Lewis base is defined as any species that can donate a pair of electrons and a Lewis acid is any species that can accept a pair of electrons. Its also a pi acceptor Lewis acid. Draw molecular geometries for the reactants and the product ofthe reaction of PF5 with the Lewis base NH3.
Trimethylborane is a Lewis acid. This indicates that they all possess acid and basic properties depending on the situation. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct.
It forms complexes with amines ethers nitriles sulfoxides and other bases. Fe is in Period 4 of the Periodic Table. The Fe atom can.
PH3 is a weaker base than NH3 because the lone pair within NH3 has a higher energy and thus serve better as a better base. Lewis acids such as boron trifluoride are so reactive that in addition to being highly volatile and toxic their pot lives are too short and exotherms too high to be useful. This product is also referred to as a Lewis adduct.
My answer is option 2 but the answer provided is 4 where am I going wrong. The groups of NH3 PH3 AsH3 and SbH3 are also amphoretic. Lewis acids and bases.
I believe that thats correct where Arrhenius and Bronsted is a reaction with water but I believe that lewis acids and bases dont necessarily have to be in water. For example NH3 is a Lewis base because it can donate its lone pair of electrons. Among the Lewis acids Compounds with incomplete or unstable electron octet as.
A Lewis base is a chemical compound that can donate a pair of electrons to a suitable electron-pair acceptor Lewis acid to form a Lewis adduct. The answer to the second question is again yes they can both behave as Lewis acids again because of expanded octets and accepting electron pairs. Upvote 0 Downvote Add comment.
As a Lewis base F accepts a proton from water which is transformed into a hydroxide ion. Therefore a Lewis base can donate a pair of electrons to a Lewis acid to form a product containing a coordinate covalent bond. It has eight valence electrons.
FeCl_3 is a Lewis acid because it can accept an electron pair from a Lewis base. Although it is well established from other answers that Phosphine is basic by generally lewis acid definitions Id like to touch on another aspect. Lewis acids and bases are described by the Lewis theory of acid-base reactions as electron-pair acceptors and electron pair donors respectively.
B CH3 3 B OH 3. Lewis acids A Lewis acid is an electron pair electrophilic that can attach electron pairs. All BrønstedLowry bases proton acceptors such as OH H 2 O and NH 3 are also electron-pair donors.
Include geometry andformal charges in the structures. Although quite reactive separately Lewis acids can form complexes. An illustration detailing the reaction between a Lewis acid and base leading to.
Lewis bases are compounds that have a free pair of electrons such as tertiary amines while Lewis acids are compounds that can accept a pair of electrons such as boron trifluoride. Behaves as a Lewis Acid showing electron-accepting properties generally in a ratio of 11 with Lewis bases. Find more Chemistry widgets in.
Thus the Lewis definition of acids and bases does not contradict the BrønstedLowry definition. It was introduced in the first half of the 20th century by Gilbert Newton Lewis. The fluorides BF3 AIF3 SIF4 and PF5 are Lewis acids.
Its electron configuration is Ar4s23d6. In FeCl_3 the three Cl atoms contribute three more valence electrons to make a total of 11.
Https Nanopdf Com Download Hsabtheory Pdf
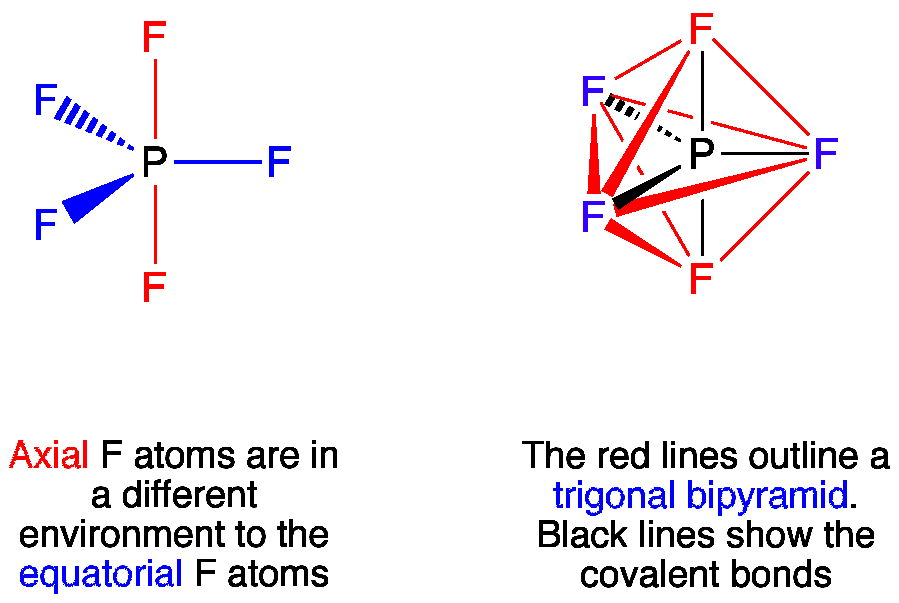
Vsepr Pf5 Phosphorus Pentafluoride
Welcome To Chem Zipper Com Lewis Acid Base Concept
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube
Phosphorus Pentafluoride Wikiwand
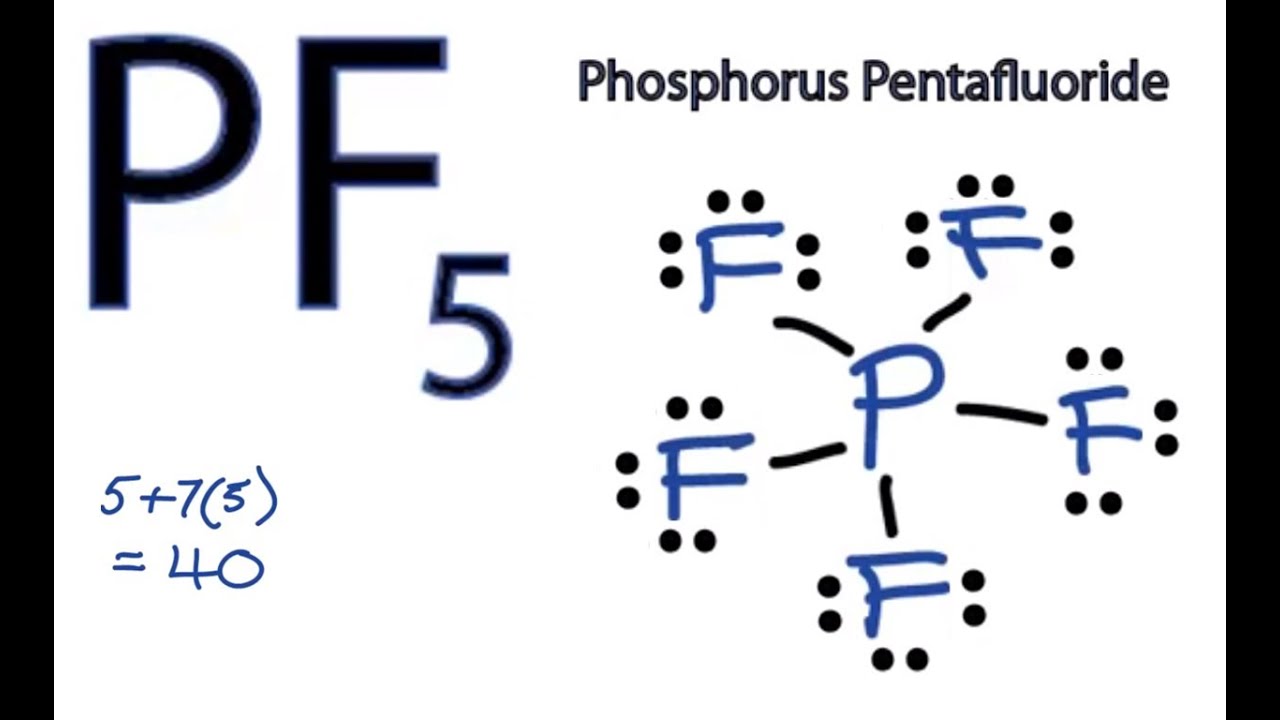
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
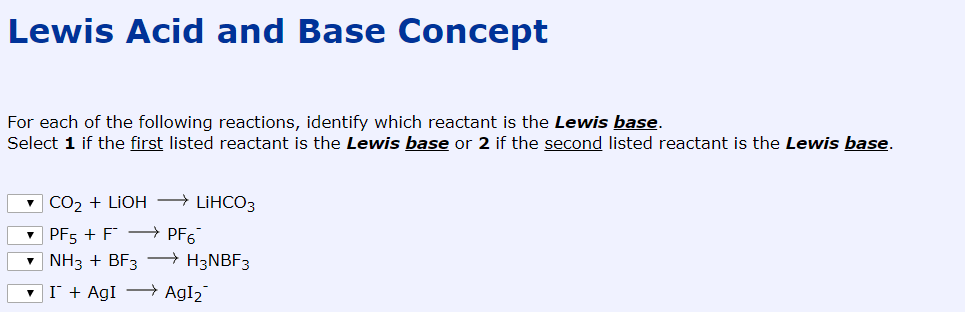
Lewis Acid And Base Concept For Each Of The Following Chegg Com

1 8 Chemistry 2810 Answers To Assignment 3 Topic Lewis

Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Incorrect Order Of Lewis Acid Character
1 Attempts Left Check My Work Select The Single Best Chegg Com
Http Www Carbene De Wp Content Cm 1111 Tutorial 6 With Answers Pdf

Phosphorus Lewis Acids Emerging Reactivity And Applications In Catalysis Chemical Society Reviews Rsc Publishing Doi 10 1039 C5cs00516g
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero


