Lewis Dot Structure For A Molecule Of Ethyne C2h2
Lewis Dot Structure of C2H2 or CHCH Acetylene or ethyne. The Lewis structure of a compound can be generated by trial and error.

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules
This is the same for the other set of CH molecules too.

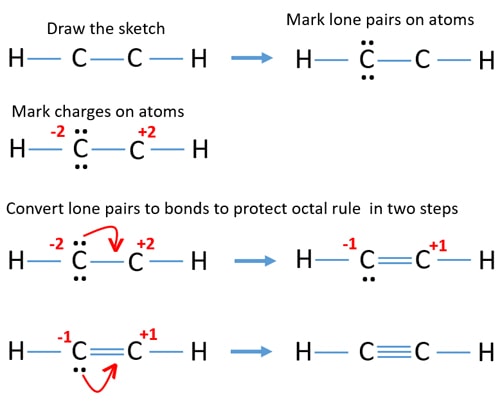
Lewis dot structure for a molecule of ethyne c2h2. To summarize this article on C2H2 Lewis structure we can say that There are ten valence electrons for Ethyne. Carbon has 4 valence electrons hydrogen has 1 valence electrons oxygen has 6 valence electrons total valence electrons 1 carbon. Hydrogen atoms only need two electrons for a full outer shell.
Carbon atom forms a single bond with one Hydrogen atom and a triple bond with another Carbon atom. Neutral Compounds practice you can also practice Lewis Dot Structures. I also go over hybridization shape sigma pi bonding and bond ang.
Since all the atoms are in either period 1 or 2 this molecule. There are no lone pairs on carbon or hydrogen atoms. Two Hydrogens are on the top and lower parts and sandwiched in btwn these two is the chained portion of carbon-carbon and the 3rd and 4th hydrogen atom.
There are no lone pairs on carbon or hydrogen atoms. The Lewis Structure For Ethyne C2h2 C2H2 Lewis Dot Structure Geometry YouTube C2H2 Molecular Geometry Shape and Bond Angles see Acetylene Wikipedia Acetylene Wikipedia. What is the Lewis structure of Hcch.
This is a total of 10. First of all find out the total number of valence electrons in the C2H2. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds.
What is the difficulty of this problem. For the HCCH Lewis structure youll need to form a triple bond between the two carbon atoms. Or if you need more Lewis Dot Structures.
C 2 H 2 Acetylene Ethyne Lewis Structure C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. In C2H2 Lewis structurethree double bonds exist between the two carbon atomsBesideseach carbon has one hydrogen atom using single bondIn C2H2 Lewis Dot structurethere are totally ten valence electrons. C 2 H 2 is a chemical formula for Ethyne a gaseous alkyne hydrocarbon.
The difference between the Lewis dot structure and the structuralformula is that the formula only shows the bonds that have formedwhereas the dot structure shows all the valen ce electronsincluding lone pairs in that molecule. Thus the Lewis structure of C2H2C2H2 is. Get more chemistry help at Electron dot structure for Ethyne C2H2.
The Lewis structures for C2H2 and C2H6 are presented below. Our tutors rated the difficulty of Draw the Lewis structure for acetylene C2H2. The structural formula for ethyne is The structural formula for ethyne is In longer alkyne chains the additional carbon atoms are attached to each other by single.
As all the valence electrons are used up in the structure there are no unshared electrons. There are a total of 10 valence electrons for the HCCH Lewis structure. 70 More Lewis Dot Structures.
C2h2 Lewis Structure C2H2 Lewis Dot Structure Geometry YouTube C2H2 Molecular Geometry Shape and Bond Angles see Hybridization Chapter 3. If you are asking help me with Lewis structures then you came to the right place for free chemistry help. I quickly take you through how to draw the Lewis Structure of CHCH Acetylene or ethyne.
HCCH Lewis Structure Ethyne is also called Acetylene. In drawing the lewis structure for c2h2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. Used in oxy-acetylene torches used for welding.
For C 2 H 2 you have a total of 10 valence electrons to work with. These electrons are known as the valence electrons. Problem 33 Medium Difficulty.
The exception of course being the. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2.
C2h2 would turn into what is known as Ethyne. Neutral Compounds practice problems. This molecule is also known by the name Acetylene.

Draw The Electron Dot Structure Of Ethyne And Also Draw Its Structure Of Ethyne Youtube

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Lewis Symbols And Structures Chemistry For Majors
C2h2 Acetylene Ethyne Lewis Structure

Draw The Lewis Dot Structure Of C2h4 C2h2 And Co2 Brainly In
Lewis Structure For C2h2 Ethyne
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Which Is The Correct Lewis Structure For Acetylene C2h2 Brainly Com

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula

Draw The Lewis Structure For Acetylene C2 Clutch Prep

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Lewis Symbols And Structures Chemistry For Majors

Lewis Symbols And Structures Chemistry For Majors

Hybridization Of C2h2 Molecular Geometry Electron Configuration Pi Bond

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

Is Co Polar Or Nonpolar Carbon Monoxide In 2021 Carbon Carbon Monoxide Polar

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube
