Lewis Structure For Pf3
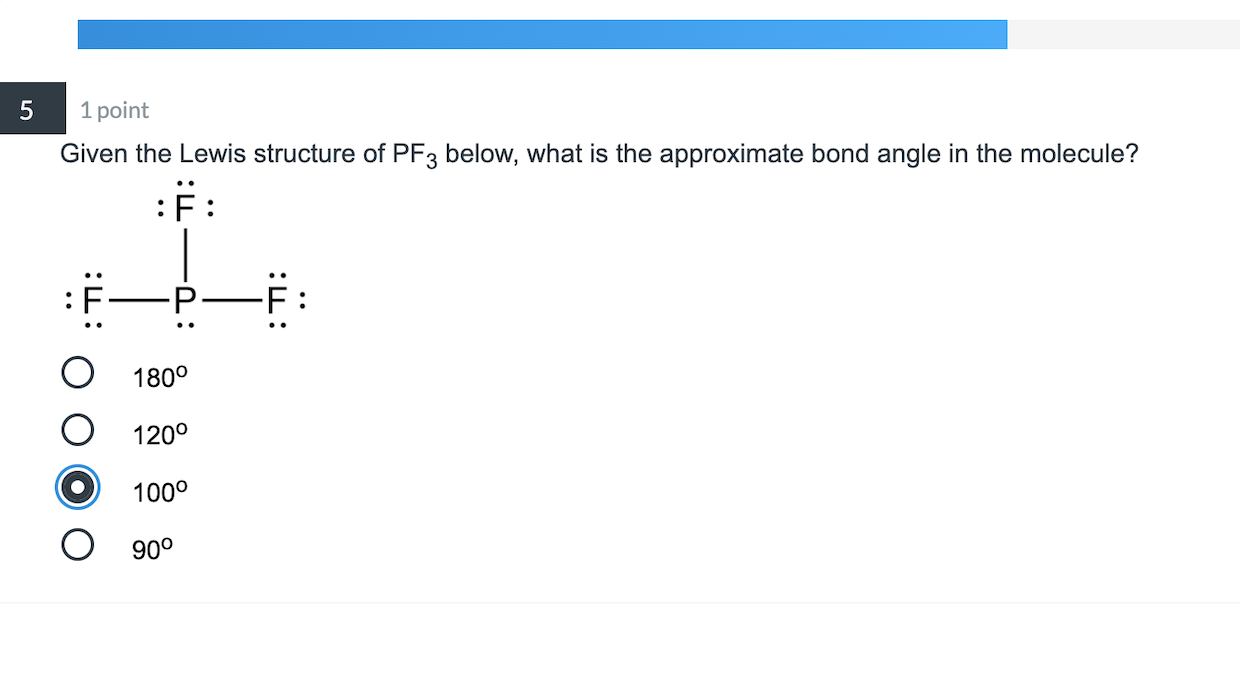
Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Get the detailed answer.
Pf3cl2 Lewis Structure How To Draw The Lewis Structure For Pf3cl2 Youtube
If we talk about the chemical composition of PF3 the molecule consists of one phosphorus atom and three fluorine atoms.

Lewis structure for pf3. Include all lone pairs of electrons. Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the structure. Drawing PF3 Lewis Structure is very easy to by using the following method.
Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21. After determining how many valence electrons there are in BF 3 place them around the central atom to complete the octets. The tetrahedral molecular geometry of CtiF will have unequal bond vectors so the molecule will have a net dipole.
The PH3 Lewis structure has 8 valence electrons. It acts as a mild Lewis acid. Draw the molecule by placing atoms on the grid and connecting them with bonds.
In the PF 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. There are a total of 24 valence electrons for the BF 3 Lewis structure. Both Lewis structures have a net formal charge of zero but note that the formal charges on the first structure are all zero.
The molecular mass of PF3 is calculated as Mol mass of PF3 1 30 mol mass of P 3 189 mol mass of F 8796 gmol. In PF3 phosphorus has one lone pair and three bond pairs which means that total electron pairs is four. 1049 a PF3- polar Draw the Lewis structure and determine the molecular geometry.
Second place the valence electron on the iodine and hydrogen atoms. Drawing the Lewis Structure for PH 3. BF3 and FeCl3 are Lewis acids.
Draw the Lewis structure for the thiocyanate ion SCN-. If you can do those Lewis structures PF 3 will be easy. We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride PF3.
Three pairs will be used in the chemical bonds between the P and F. 1 on a question. It has sp3 Hybridization and the bond angle is approximately 1095.
B Determine the oxidation numbers of the P a Free unlimited access for 30 days limited time only. Click to see full answer. 1 To draw the Lewis structure of PF3 P F 3 we first count for the valence electrons of the compound.
Correspondingly what Vsepr shape is pf3. Thus the first Lewis structure is predicted to be more stable and it is in fact the structure observed experimentally. There are a total of 26 valence electrons for PBr3.
What are the total numbers of bonds and nonbonding electrons for PF3. The molecule is trigonal pyramidal-shaped and is a polar molecule. In this post we discussed the method to construct the CH3I Lewis structure.
PF3 though it has a filled s and p orbitals it can accept electrons from donors and expand its coordinate number up to six due to vacant d orbitals. What is the hybridization of PF3. What is the molecular shape of PF3.
In general the closer the formal charges are to zero the more stable the structure. In the Lewis structure for PF 3 there are a total of 26 valence electrons. O Bent O Tetrahedral O Linear O Trigonal planar The P-F bond in PF3 ispolar Trigonal pyramidal The molecule PF3 is polar What is the F-P-F bond angle.
Three single covalent bonds are formed between the phosphorus and fluorine atoms which contributes to the presence of three strong sigma bonds and no pi bonds. PF 3 is similar to PBr 3 and PCl 3. The valence electrons of Phosphorus are 5 and fluorine has 7 valence electrons in its outermost shell.
Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. What are the electron-pair geometry and the molecular geometry of the central atom for SCN. For the BF 3 Lewis structure calculate the total number of valence electrons for the BF 3 molecule.
PF3 has 26 valence electrons. Draw the Lewis dot structure for PF3. The molecular geometry from Exercise 35 is trigonal pyramidal.
Drawing the Lewis Structure for PF 3. First the valence electrons are placed around the carbon atom. The Lewis structure of the tetra-atomic phosphorus trifluoride PF3 molecule shows three fluorine atoms bonded to a single phosphorus central atom.
The phosphorus trifluoride chemical formula is PF3. For the PH3 Lewis structure we first count the valence electrons for the PH3 molecule using the periodic table. Draw the Lewis structure for PF3.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Draw a Lewis structure for the molecule. Here in this post we described.
Write a Lewis structure for the phosphorus trifluoride molecule PF3. PF3 Draw the Lewis structure for PF3 in the box at the right including lone pairs. A Draw the dominant Lewis structure for the phosphorus trifluoride molecule PF3.
In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom.

Lewis Structure For Pf3 Learn Lif Co Id

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube
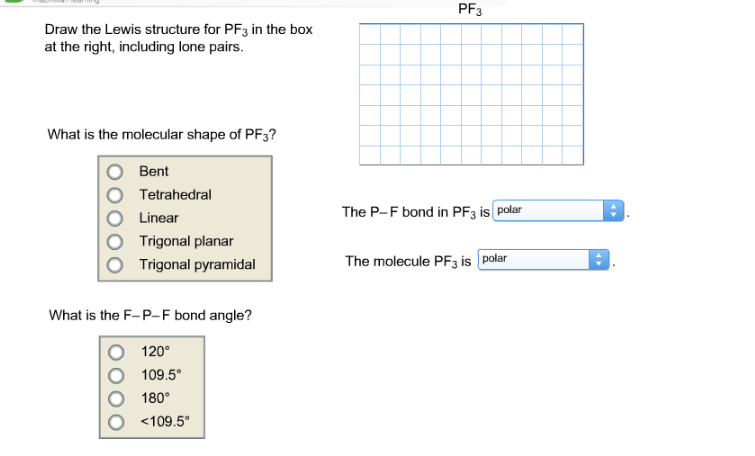
Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Pf3 Lewis Structure Lewis Structure Of Pf3 Phosphorus Trifluoride Draw Lewis Structure For Pf3 Youtube
5 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com

9 3 Drawing Lewis Structures Chemistry Libretexts

What Is The Molecular Geometry Of Pf3 Study Com

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube

What Is The Lewis Structure For Pf3 Study Com
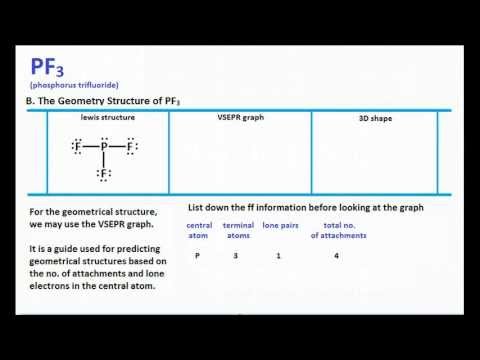
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

A What Is The Molecular Geometry Of Pf3 Clutch Prep
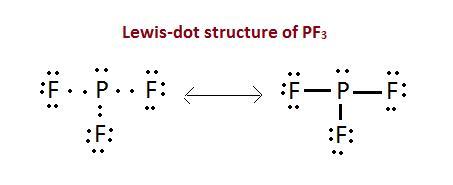
The Lewis Structure Of Pf3 Shows That The Central Phosphorus Atom Has Nonbonding And Bonding Electron Pair S

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube
Lewis Dot Structure And Vsepr Model Sophat

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist