N2 Lewis Structure Molecular Geometry
Select b How many lone pairs of electrons are present on the central atom in the Lewis structure. N2 lewis structure bond bonds form setup lone science each three atom hard easy atoms needs pair times.

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Molecular Shapes Chemistry Help
Metals in an ionic bond give what to nonmetals.
N2 lewis structure molecular geometry. Start by calculating how many valence electrons youd get for a molecule of nitrogen gas N2. 031920 This problem has been solved. However this has several forms of existence as chemical entities.
As a neutral compound this is known to exist as a radical known as amino radical and therefore has the formula NH2. The Valence Shell Electron Pair Repulsion theory or VSEPR theory is one useful theory for predicting the geometries of molecules. The octet rule states that an atom should have eight electrons in its outer shell to be stable.
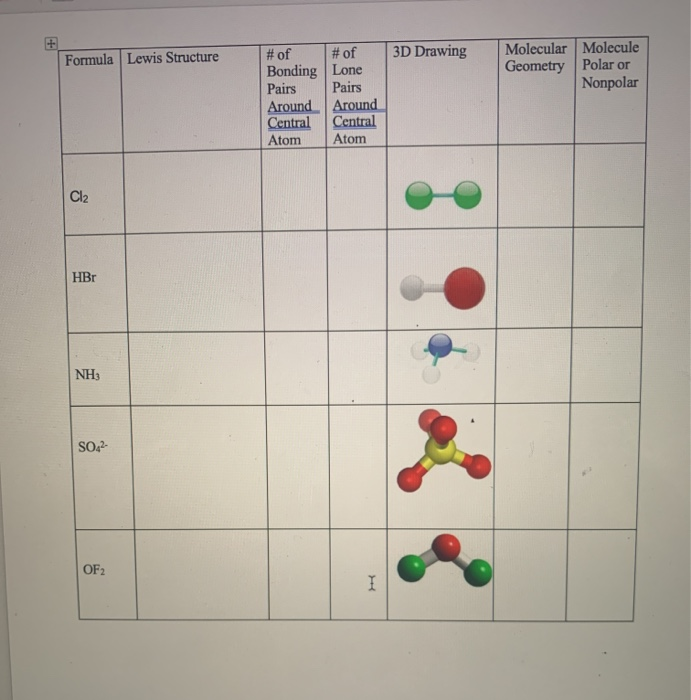
031920 H2 N2 CO CO2 CO32- Last Modified. Both oxygens are attached to the carbon. Molecular Geometry Lab Molecule Lewis Dot Structure Incorporating Bond Polarity Molecular Geometry Lewis Dot Structure Incorporating Geometry Resonance YES OR NO Overall Polar OR Non-Polar SF 4 SiF 2 H 2 SO 2 CS 2 caff F I 9 E is sawn 4 crooks Np Polar s seasaw FOI p L F H H lek E it E tetra Fung No Polar 20 ve fl B c hedral f f H up p H.
An explanation of the molecular geometry for the N2 ion Nitrogen gas including a description of the N2 bond angles. 31 N2 Lewis Structure Molecular Geometry Background. The first is nitrogen dioxide which is a byproduct of many industrial processes and is present in car exhaust.
There is a triple bond. In order to be able to determine the molecular geometry of a given compound you need to first draw its Lewis structure. NH2 Lewis Structure Molecular Geometry Hybridization and Polarity NH2 as we can see is a chemical composition of nitrogen and hydrogen atoms.
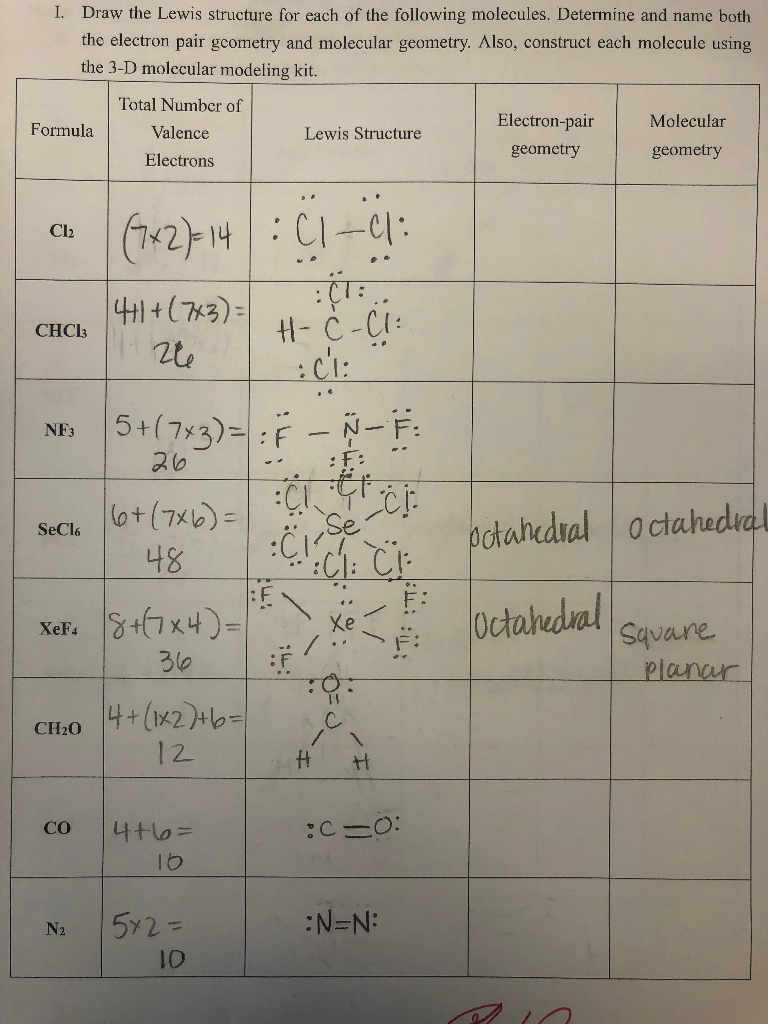
5 rows N2 Lewis structure would comprise of two atoms of Nitrogen N atoms. Total of Molecular Formula Lewis Structure valence e shape around central atoms Ci - trigonal H H CN H CN-H 31 4 planar H H 1 2 5 12 N2 - bent 15 pts 025 pts 125 pts OH HOCNH 15 pts 025 pts 125 pts H3CCOOH Hint. Nitrogen gas N2 N 2 has a triple bond between the two nitrogen atoms.
Select c What is the molecular geometry of OF2. H2 N2 CO CO2 CO32- Last Modified. Terms in this set 46 7 diatomic molecules.
Atomic symbols in a Lewis dot structure represent nucleus. Transcribed image text. Lewis Structures Molecular Geometry and bonding.
5 rows In the lewis structure of N2 there is a triple bond between two nitrogen atoms. The molecular orbital diagram for nitrogen is as follows You can see the accounting for each of the valence electrons 5 from each atom place. N2 lewis structure molecular geometry hybridization bond nitrogen covalent electrons triple distribution.
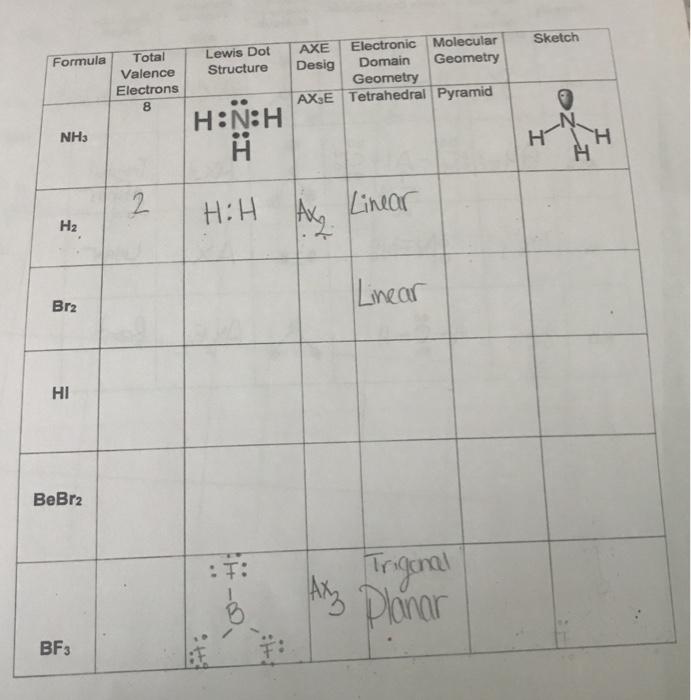
A How many bonding pairs of electrons are in the Lewis Structure. Lewis Structure of Valence Electrons Molecular Geometry Name and Sketch Polar or Nonpolar. The Lewis structure of this substance is shown below.
Nitrogen is located in period 2 group 15 of the periodic table which tells you that it has 5 valence electrons. H2 N2 O2 F2 Cl2 Br2 I2. Draw the correct Lewis structure of OF2 and then use it to answer the questions below.
Experiment 12 Lewis Dot Structures and Molecular Geometry 12-2 Procedure for Determining Geometry Once the Lewis structure of a molecule or ion is determined the 3-D shape of the molecule can be determined. Structure lewis n2 nitrogen dot diagram electron ch2o gas electrons science hard bonds triple easy example double. N2 Lewis Structure Shape n2 lewis structure molecular geometry hybridization bond nitrogen covalent electrons triple distribution n2 nitrogen lewis structure dot gas draw diatomic n2 lewis structure bonds bond pair each atom atoms form setup lone science three hard needs times n2 geometry structure lewis molecular hybridization polar nonpolar nitrogen atoms vsepr.
The electron geometry for the Nitrogen. Each atom also has 1 lone pair. The Lewis structure for Nitrogen is as follows You can see the triple bond with the lone pair on both of the nitrogen atoms.
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape

Drawing Complex Lewis Structures And Formal Atom Charges University Aid Covers How To Draw Electron Dot High School Science Secondary Science Teaching Science
3d Drawing Formula Lewis Structure Molecular Chegg Com
Draw The Lewis Structure For Each Of The Following Chegg Com
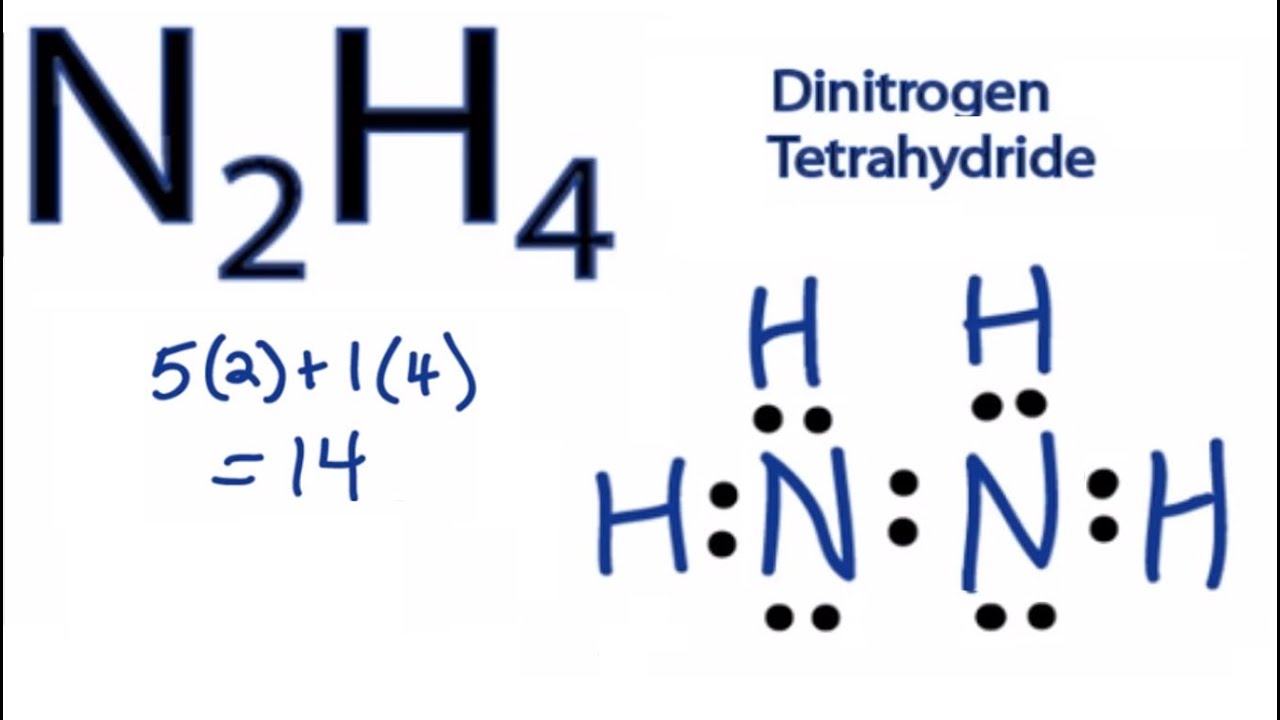
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
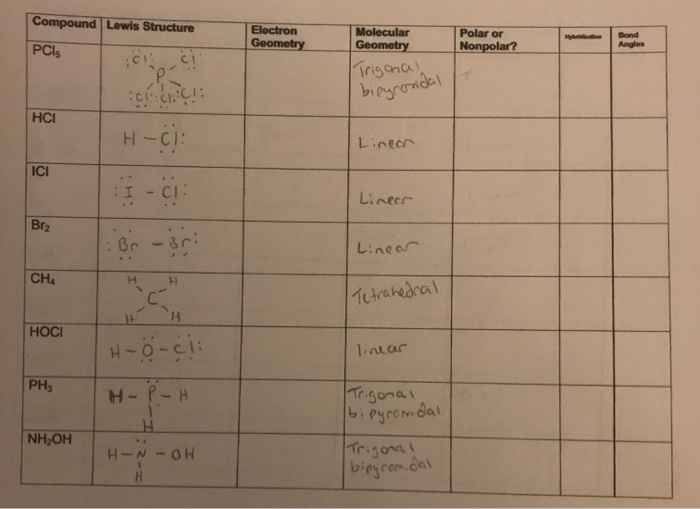
Compound Lewis Structure Electron Geometry Polar Or Chegg Com

N2 Lewis Structure Molecular Geometry And Hybridization Techiescientist

N2h2 Molecular Geometry Bond Angles And Electron Geometry Youtube

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

N2 Lewis Structure Hybridization Molecular Geometry What S Insight

Lewis Dot Structure And Molecular Geometry For N2f4 Tetrafluorohydrazine Youtube

N2 Nitrogen Gas Molecular Geometry And Bond Angles Youtube
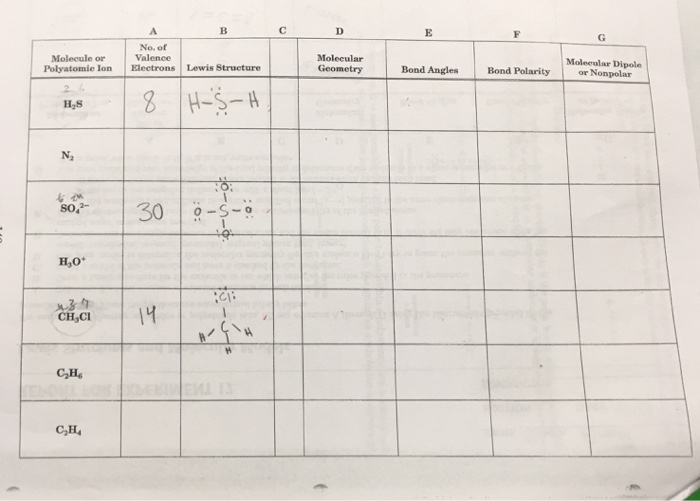
No Of Molecule Valence Molecular Geometry Molecular Chegg Com
Sketch Formula Lewis Dot Structure Total Valence Chegg Com

How To Draw The Lewis Dot Structure For C2n2 Cyanogen Youtube

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Chemistry Help Molecular Shapes

N2 Nitrogen Gas Molecular Geometry And Bond Angles Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas
H2s Lewis Structure Molecular Geometry Hybridization And Polarity