Ncl3 Lewis Structure Polar Or Nonpolar
Steps to Identify Polar Molecules. Is NCl3 Polar or Nonpolar.
Lewis Structures And Molecular Structure
Learn to determine if BCl3 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then us.
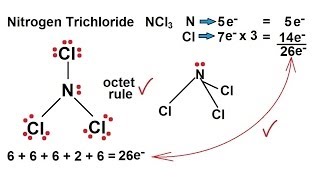
Ncl3 lewis structure polar or nonpolar. The steric number of iodine central atom in the ICl2- the molecule is 5 thus it forms Sp 3 d hybridization. Figure out the geometry using VSEPR theory Visualize or draw the geometry. For NCl3 draw the Lewis structure predict the shape and determine if the molecule is polar or nonpolar.
NCl3 is a slightly polar molecule. Cl3 is polar because of its trigonal pyramidal structure. If possible try to say if its symmetrical or asymmetrical and in the end if you know if its a polar or a non-polar please do tell.
Interhalogen compounds are molecules which contain at least two different halogen atoms. Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. 1 There is a net dipole moment but it is very weak since the electronegativity difference between nitrogen and each of the chlorine atoms is 316 304 012.
Otherwise it is polar. H 2 O m. For NCl3 draw the Lewis structure predict the shape and determine if the molecule is polar or nonpolar.
Yes it is non polar. CF 2 H 2 e. CCl 2 F 2 d.
NCl3 is a slightly polar molecule. One of the examples for a non polar molecule was Tetrachloromethane CCl4. The nature of ICl2- is nonpolar because all dipole that generated along the bond will cancel out because of.
NCl3 N C l 3 is a polar molecule. CH 2 O f. Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules.
To explain why we can refer to its Lewis structure below. PCl5 is a water-sensitive colorless and moisture. Notice that a tetrahedral molecule such as ceCCl_4 is nonpolar â please describe or explain all the necessary things such as the electronegativity and if the.
Draw the Lewis structure. Is NCl3 polar or non-polar. NCl3 molecule has one lone pair that leads to repulsion between electrons and the shape of the molecule is trional pyramidal.
NCl3 is a polar molecule due the presence of a lone pair of electrons. Nitrogen trichloride is a yellow oily liquid with its pungent odor. N 2 O i.
Ill tell you the polar or nonpolar list below. This is because nitrogen has a lone pair of electrons that repels the bonded electron pairs of the N-Cl covalent bonds thus giving the molecule an asymmetric structure where the polarities of the bonds do not cancel each other out. Find the net dipole moment you dont have to actually do calculations if you can visualize it If the net dipole moment is zero it is non-polar.
This leads to electron-electron repulsion which results in a bent structure thereby causing an unequal distribution of charge within the molecule and creating a permanent dipole. These compounds are generally written as ABn where n 1 3 5 and 7. N C l 3 has the same geometry as ammonia which is pyramidal with a C 3 v point group.
CCl 2 F 2 d. CH 2 O f. If the electronegativity of all three are the same then NCl3 will be non-polar.
ICl3 Lewis Structure Molecular Geometry Hybridization and Polarity. Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no. N 2 O i.
Ncl3 lewis structure polar or nonpolar - optimumge. If you look at the Lewis structure for POCl3 we can see that it is not a symmetrical molecule. NCl3 is slightly polar because the lone pair present on the nitrogen generates repulsion between electrons makes the overall structure bent and this creates unequal charge distribution of charge within the structure that generates a permanent dipole moment.
NCl3 is a slightly polar molecule because of the small difference between the electronegativity of nitrogen and chlorine atom. The total valence electron is available ICl2- lewis structure is 22. From the above we can see that the central nitrogen atom has a lone pair.
CF 2 H 2 e. ICl3 named Iodine Trichloride is an Interhalogen compound. A and B are less electronegative and more electronegative elements respectively.
03 - 17 polar covalent.

Brcl3 Lewis Structure How To Draw The Lewis Structure For Brcl3 Bromine Trichloride Youtube
4 2 Lewis Structures Problems Chemistry Libretexts
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube
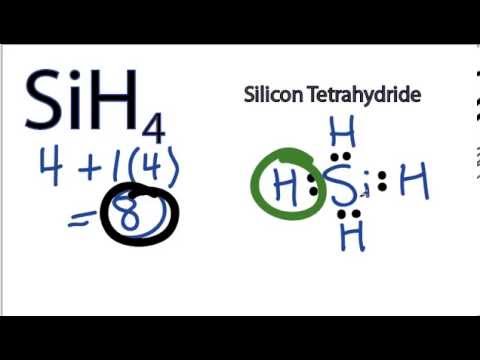
Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
Solved 7 Describe The Shape And Give Lewis Structures That Suggest The Shapes Of The Following A Ccl2f2 Dichlorodifluoromethane B Nf3 Nitrogen Course Hero

Lewis Structure Of Ncl3 Nitrogen Trichlorode Youtube

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Using Formal Charges To Evaluate Nonequivalent Resonance Structures Worked Example Video Khan Academy

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Https Www Birmingham K12 Mi Us Cms Lib Mi01908619 Centricity Domain 1854 Unit 203 20 20assignment 208 20 20lewis 20structures 20vsepr 20polarity 20remix 20 20answer 20key 20complete Pdf

Lewis Structure Of Po3 1 Simple Method For Lewis Electron Dot Structures Context Clues Worksheets High School Chemistry Chemistry

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry
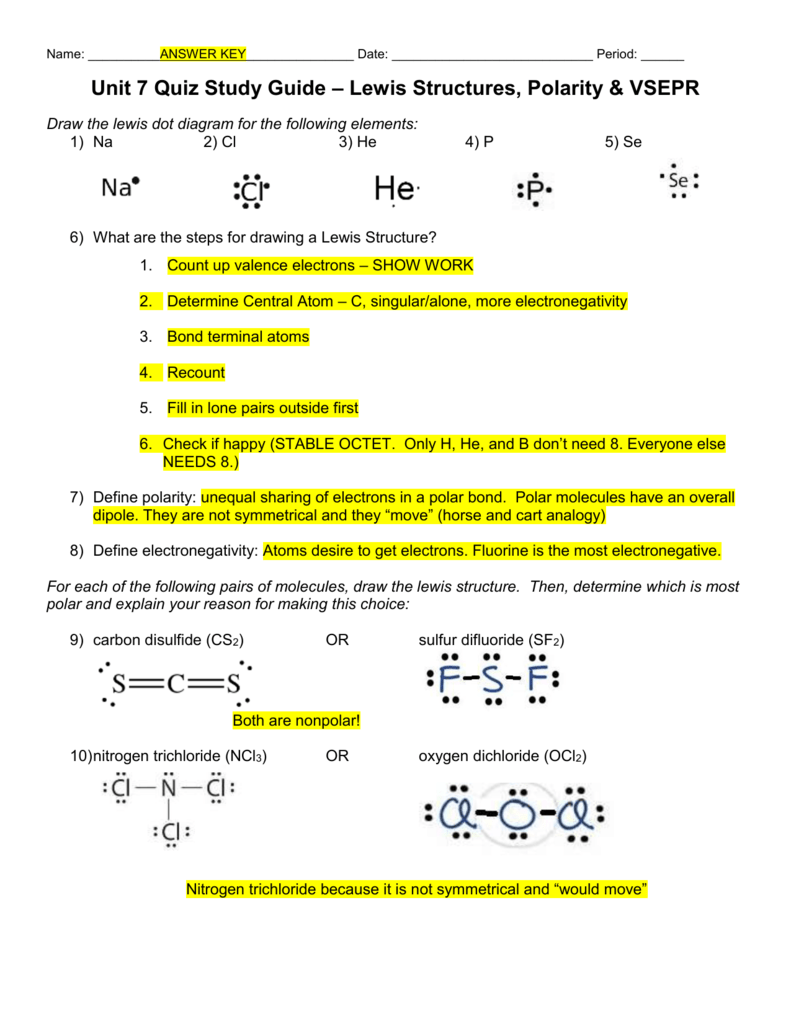
Unit 7 Quiz Study Guide Lewis Structures Polarity Vsepr

No2 Lewis Structure How To Draw The Lewis Structure For No2 Youtube
Question 9 2 Pts What Is The Formal Charge Of The Chegg Com