Nitrogen Trifluoride Lewis Dot Structure
Were you searching for a short yet detailed video on NF3 Lewis Structure. Each fluorine atom has three lone pairs.

Ch3cl Lewis Structure Chloromethane In 2021 Lewis Molecules Methylation
How many atoms of Care in 0185 mol of C3H52O.

Nitrogen trifluoride lewis dot structure. Nitrogen fluoride oxide F3NO CID 26304 - structure chemical names physical and chemical properties classification patents literature biological activities. In this case those are Nitrogen and Fluorine. Get the detailed answer.
Which is the correct Lewis structure for nitrogen trifluoride NF3. Which is the correct Lewis structure for nitrogen trifluoride. Its noticeable characteristics include being colorless and carrying a musty or moldy odor.
1 Correct skeleton structure of the compound should be written first. Add 1 electron for each negative charge and subtract 1 electron for each positive charge. The Lewis dot structures of the individual non-metal atoms give a good indication of the bonding possibilities for the atoms.
It also explains why the molecule is not drawn as flat but rather has a pyramidal look to it. The total valence electron available for the Nitrogen trifluoride lewis structure is 26. A video explanation of how to draw the Lewis Dot Structure for Nitrogen Trifluoride along with information about the compound including Formal Charges Pola.
0 Response to Lewis Dot Diagram For Nitrogen Trifluoride Post a. A video explanation of how to draw the Lewis Dot Structure for Nitrogen Trichloride along with information about the compound including Formal Charges Pola. NF3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble.
Nitrogen tends to form three bonds and have on e lone pair. Carbon tends to form 4 bonds and have no lone pairs. Write a Lewis structure for nitrogen trifluoride NF3.
Calculate the molar mass of MgPO42. Nitrogen trifluoride appears as a colorless gas with a moldy odor. A step-by-step explanation of how to draw the NI3 Lewis Dot Structure Nitrogen TriiodideFor the NI3 structure use the periodic table to find the total num.
Lewis dot structure of NF3. 1 Draw skeletal structure with the central atom being the least electronegative element. 2 Sum the valence electrons.
In the NF 3 Lewis structure and all structures hydrogen goes on the outside. Hybridization of NF3 is Sp³. Draw Lewis Structures For Each Of The Following 1 Nitrogen.
Very toxic by inhalation. The molecular geometryVSEPR shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral. Ncl3 Dot Diagram Wiring.
A step-by-step explanation of how to draw the NF3 Lewis Dot Structure Nitrogen trifluorideFor the NF3 structure use the periodic table to find the total n. In the lewis structure of Nitrogen trifluoride NF 3 there are three N-F bonds and one lone pair on nitrogen atom. What is the mass percent.
4 Complete electron octets for atoms bonded to. NF3 is polar in nature. If yes then we have got you.
By looking at the Lewis Dot structure of Nitrogen Trifluoride we can understand how it takes on its triangular shape as seen to the here. 3 Subtract 2 electrons for each bond in the skeletal structure. Ncl3 Dot Diagram Wiring.
2 After writing the skeleton structure total number of electrons should be calculated by adding the valence electrons. What Is The Lewis Dot Structure For Nitrogen Chlorine Quora. NF 3 Nitrogen trifluoride is very similar to the NCl 3 and NH 3 Lewis structure.
Each step of drawing the lewis structure of NF 3 is. Oxygen tends to form two bonds and have two lone pairs. Slightly soluble in water.
Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Today in this video we will help you determin.
To write the Lewis structures for molecular compounds one must follow the following steps. Hydrogen only needs two valence electrons to have a full outer shell. Name the shape and state whether the molecule is polar or non-polar.
In the Lewis structure for NF 3 there are a total of 8 valence electrons. To build a picture of Nitrogen Trifluoride we need to start with the electron dot diagrams for the elements involved in the molecule. NF3 has a molar mass of around 71002 gmol and a density of 3003 kgm3.
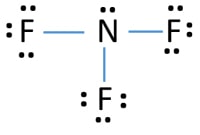
Nf3 Nitrogen Trifluoride Lewis Structure Steps Of Drawing

Select The Correct Lewis Structure For Nit Clutch Prep

Drawing Lewis Structures Chemistry Socratic
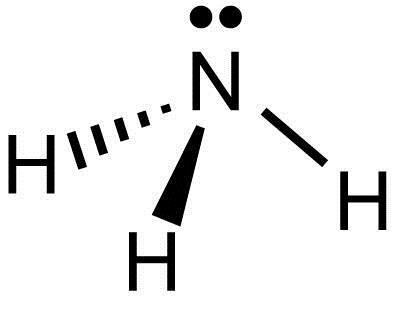
What Is The Lewis Structure Of Nh3 Socratic

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Lewis Dot Structure For Hydrogen H Lewi Electron Configuration Chemistry Worksheets Chemical Equation

Lewis Structure For Bf3 Boron Trifluoride

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Lewis Symbols And Structures Chemistry For Majors

Nf3 Lewis Structure Molecular Geometry Polarity Bond Angle Hybridization

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Select The Correct Lewis Structure For Nit Clutch Prep
Nh3 Lewis Structure Ammonia Youtube

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Nf3 Lewis Structure Molecular Geometry Polarity Bond Angle Hybridization
Does It Matter Where You Put The Dots On A Lewis Structure Quora
Diagram Lewis Dot Diagram Nf3 Full Version Hd Quality Diagram Nf3 Diagramrt Bmwe21fansclub It