Nitrogen Triiodide Lewis Structure
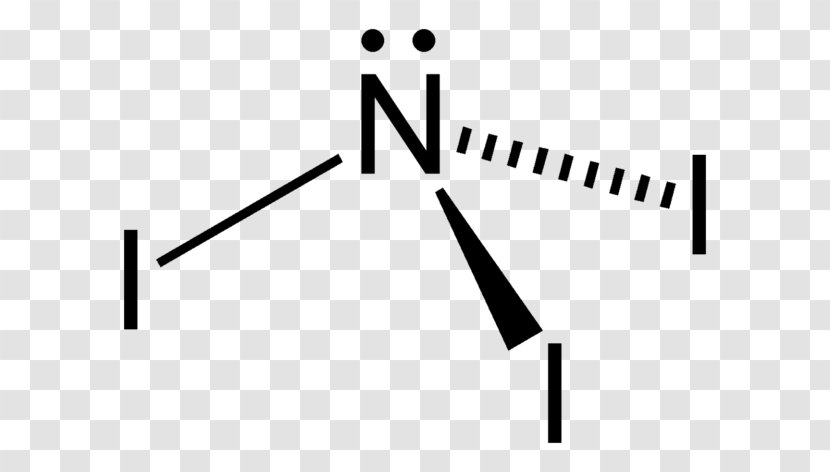
In the reaction the iodine atoms are displacing the hydrogen atoms in ammonia as shown. The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry.

How To Draw Lewis Structure Of Ni3 Drawing Easy
Weve used all 26 valence electrons.
Nitrogen triiodide lewis structure. How is the Lewis structure for nitrogen dioxide drawn. In which 1 atom of Nitrogen combines with 3 atoms of Iodine. So that is the Lewis structure for NI3.
For the n3 lewis structure use the periodic table to. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Both Nitrogen and Iodine are non-metalsSo they form covalent bond by sharing of electrons.
Nitrogen Triiodide and Lewis Structures. NO2 has 56617 valence electrons. Lewis structure for nitrogen triiodide is given in the attachment.
The electron configuration of Nitrogen and Iodine is given below. Each of the Is s has eight valence electrons so the octets are satisfied there. N is in the center and sp2 hybridized to a triangular planar shape.
The given compound is Nitrogen triiodide. You could also draw it like this right here as a structural formula. We would like to show you a description here but the site wont allow us.
Its a covalent molecule which means the atoms in the molecule share electrons. The chemical formula NI 3 is named nitrogen triiodide. Presents dangerous fire hazard in the presence of reducing agents.
Nitrogen triiodide is highly unstable compound due to the weak bonds of the nitrogen to the iodine atoms and the size difference nitrogen. Etches glass in the presence of moisture. One O atom is double bonded and has two nonbonded electron pairs on it.
The iodine reacts with ammonia to produce the nitrogen triiodide ammoniate in its stable form. When we draw resonance structures we convert lone pairs to bonds and bonds to lone pairs when it is possible. As it dries the compound loses its stability due to the formation of the nitrogen gas and causes an easy explosion.
The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry. The chemical formula NI 3 is named nitrogen triiodide. And then in the center the Nitrogen also has 8 valence electrons so those octets are satisfied.
NITROGEN TRIFLUORIDE is a very powerful oxidizing agent. The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair. Lets see if we have octets.
Emits toxic and corrosive fumes of fluoride when heated to decomposition Lewis 3rd ed 1993 p.
Lewis Structure Nitrogen Triiodide Ammonia Amine Watercolor Transparent Png
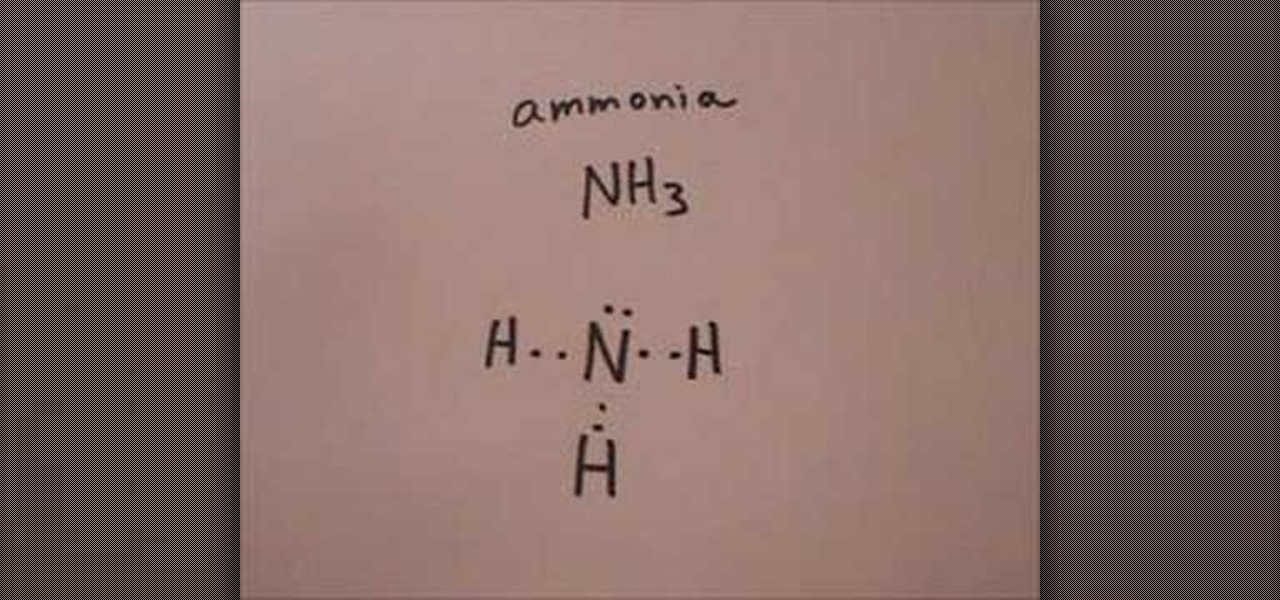
How To Draw The Lewis Structure For Ammonia Science Experiments Wonderhowto

What Is The Lewis Structure Of Nitrogen Triiodide Brainly Com

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

How To Write The Formula For Nitrogen Triiodide Youtube
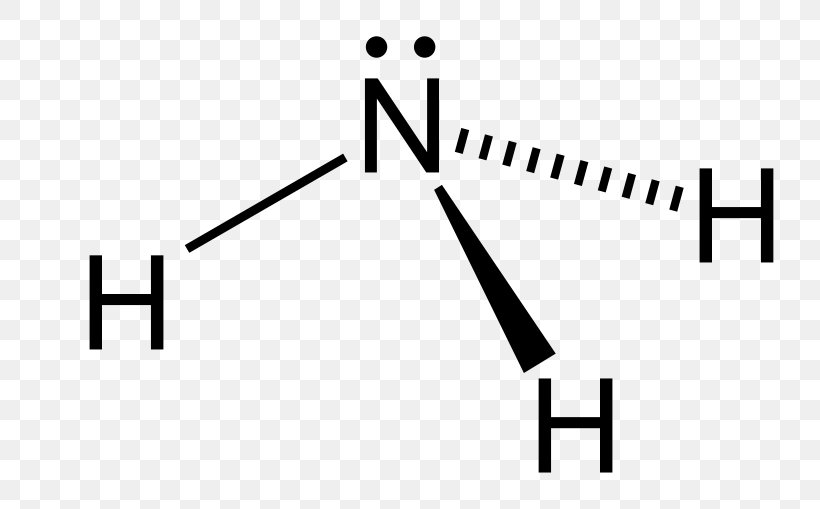
Lewis Structure Nitrogen Trifluoride Nitrogen Triiodide Molecular Geometry Ammonia Png 800x509px Lewis Structure Ammonia Area Atom

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
When A Covalent Lewis Structure Is Drawing Using One Nitrogen N How Many Lodine Atoms Will Bond To The Nitrogen Quora

Chemical Bonding Covalent Bonds Ppt Download

Chapter 8 And 9 Notes Ppt Download
Nitrogen Triiodide Ni3 Pubchem

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Molecular Geometry Ck 12 Foundation

What Is The Lewis Structure Of Ni3 Study Com

I3 Lewis Structure Triiodide Youtube
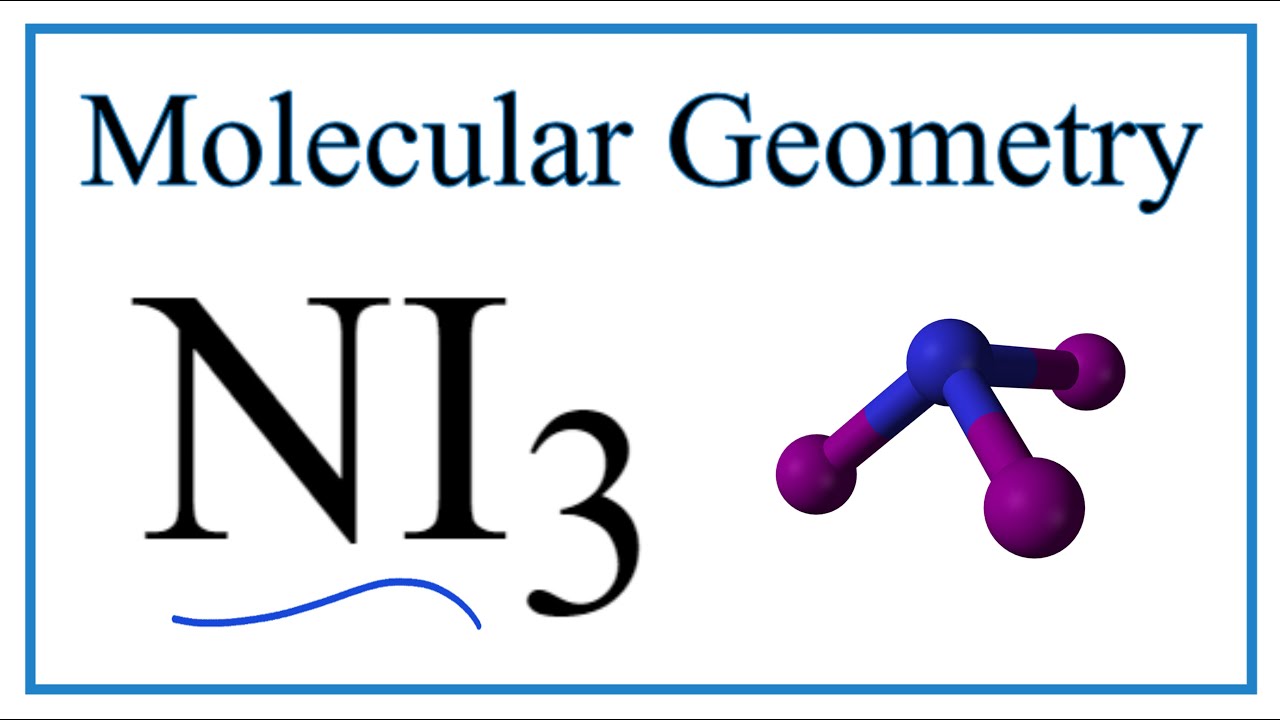
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube
