Pf3 Lewis Structure Valence Electrons
The molecular mass of PF3 is calculated as Mol mass of PF3 1 30 mol mass of P 3 189 mol mass of F 8796 gmol. Phosphorus has 5 valence electrons in its outer shell.

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Here in this post we described.

Pf3 lewis structure valence electrons. Three pairs will be used in the chemical bonds between the P and F. PF 3 is similar to PBr 3 and PCl 3. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095.
Second place the valence electron on the iodine and hydrogen atoms. In the PF 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21.
Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. If you can do those Lewis structures PF 3 will be easy. PF3 has 26 valence electrons.
A step-by-step explanation of how to draw the PF3 Lewis Dot Structure Phosphorous trifluorideFor the PF3 structure use the periodic table to find the tota. Draw a Lewis structure for the molecule. The phosphorus trifluoride chemical formula is PF3.
Solution for Formula Lewis structure Molecule or Electron group Molecular Bond Polarity Ion Type geometry geometry angle PF3 H2O PF3. In this post we discussed the method to construct the CH3I Lewis structure. To draw the Lewis structure of PF3PF3 we first count for the valence electrons of the compound.
Select Erase Rings Draw More F P. The Lewis structure of PF3 shows that the central phosphorus atom has how many non bonding and how many bonding electron pairs. Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation.
1 To draw the Lewis structure of PF3 P F 3 we first count for the valence electrons of the compound. Total number of valence electrons 5 35. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition.
PF3 has 26 valence electrons. PF3 has 26 valence electrons. 41 The Lewis structure of PF3 shows that the central phosphorus atom has __________ nonbonding and __________ bonding electron pairs.
So weve used 26 valence electrons that we started with. You can see the central S atom has 4 bonding. The valence electrons of Phosphorus are 5 and fluorine has 7 valence electrons in its outermost shell.
Drawing PF3 Lewis Structure is very easy to by using the following method. If we talk about the chemical composition of PF3 the molecule consists of one phosphorus atom and three fluorine atoms. PF3 has 26 valence electrons.
C r -p -F Determine the total number of electron. Drawing the Lewis Structure for PF 3. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot.
So we have 35 valence electrons for all Fluorine atoms. Draw a Lewis structure for the molecule. Fluorine has 7 valence electrons in its outer shell but as there are 5 fluorine atoms we will multiply the number by 5.
First the valence electrons are placed around the carbon atom. Include all valence electrons. Draw a Lewis structure for the molecule.
Because they are present in the outermost shell the hold of the nucleus is weak on them. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation.
Draw a Lewis structure for the molecule. Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21 valence electrons 7 for each F atom giving a total of 26 valence electrons. 41 The Lewis structure of PF3 shows that the central.
In the Lewis structure for PF 3 there are a total of 26 valence electrons. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095.

What Is The Molecular Geometry Of Pf3 Study Com
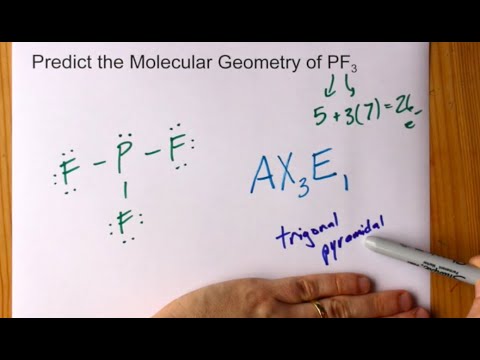
Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube

How To Draw The Lewis Dot Structure For Pf4 Youtube

Pf3 Lewis Structure Lewis Structure Of Pf3 Phosphorus Trifluoride Draw Lewis Structure For Pf3 Youtube
Pf3cl2 Lewis Structure How To Draw The Lewis Structure For Pf3cl2 Youtube

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Bf4 Lewis Structure How To Draw The Lewis Structure For Bf4 Youtube

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

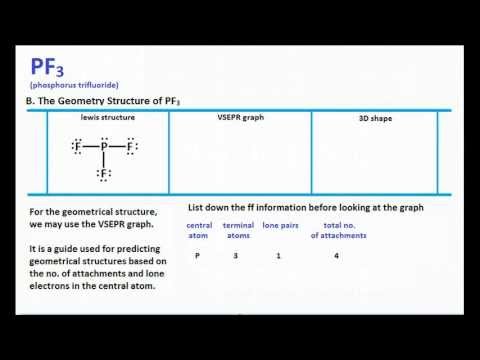
Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
Lewis Dot Structure And Vsepr Model Sophat
Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube