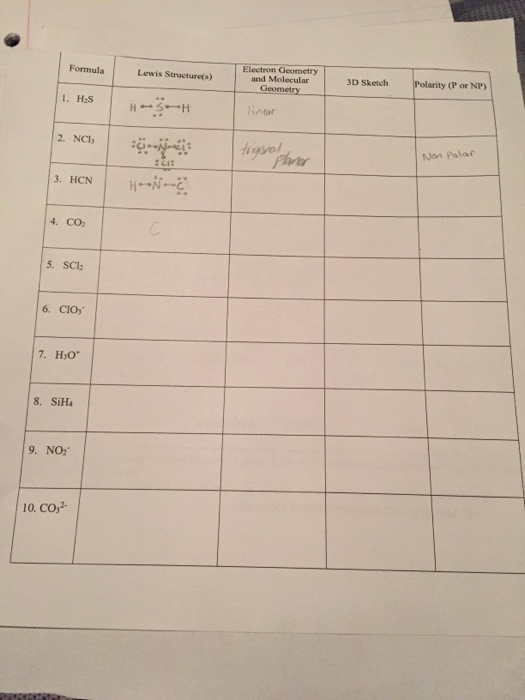
Scl2 3d Structure
Molecular Structure Cont VSEPR Intro. Start with the molecules Lewis structure which is drawn like this.

Is Scl2 Polar Or Nonpolar Techiescientist
Make sure you have the latest version of Java installed and that it is enabled on your browser.
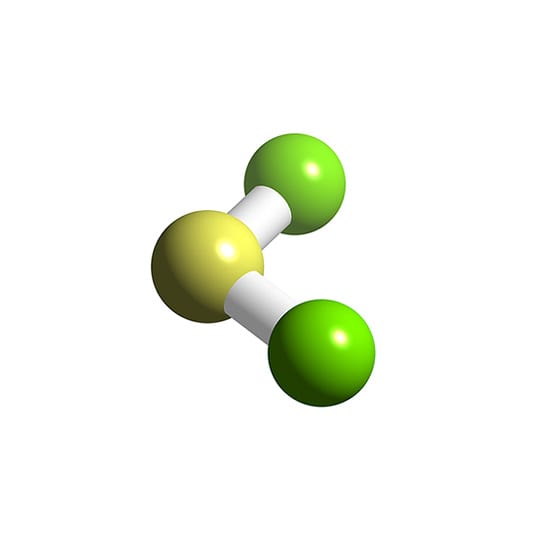
Scl2 3d structure. The product contains about 72 - 80 SCl2 residual S2Cl2 and Cl2. Its hybridization is sp3 because SCl2 doesnt require pi bonding. Beryllium chloride is a white to green solid with a sharp odor.
Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70. The radius of the spheres is therefore smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of SULFUR DICHLORIDE. S 2 Cl 2 Cl 2 2 SCl 2.
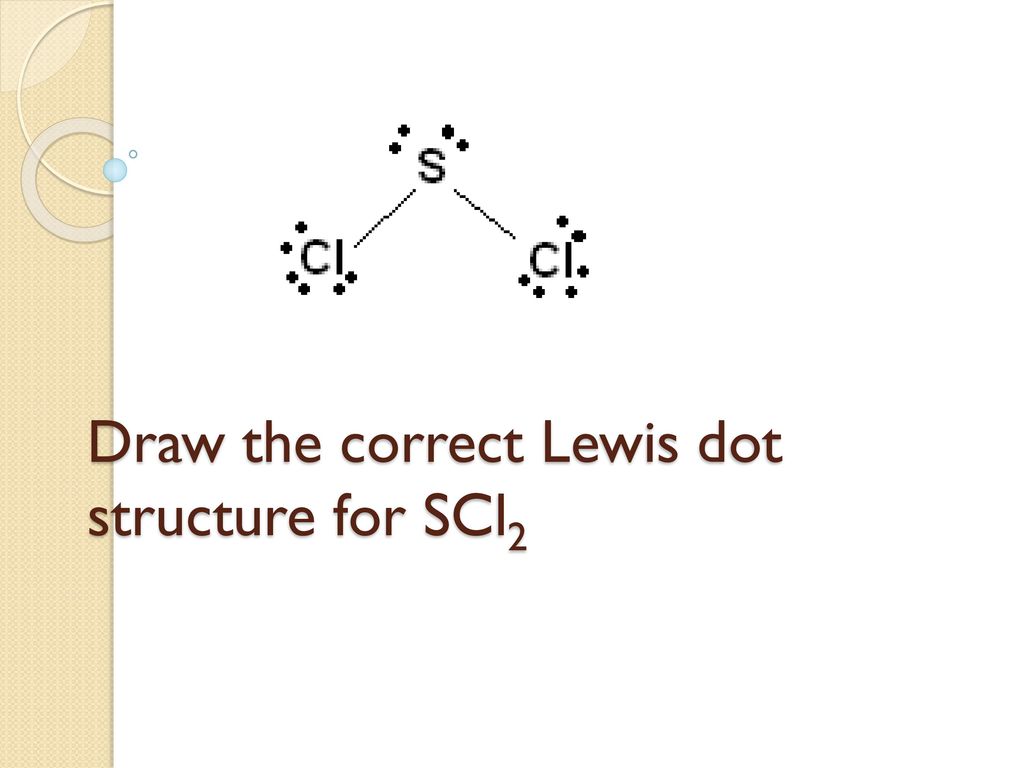
What Is The Lewis Structure And VSEPR 3D Structure show Polarity Of SCl2. Structures 2 and 3 in the example above are somewhat fictional structures in that they imply that there are real double bonds and single bonds in the structure for ozone. There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure.
What is the Lewis structure and VSEPR 3D structure show polarity of SCl2. S2 is bonded in a water-like geometry to two Cl1- atoms. Beryllium dichloride is a compound of beryllium 2 oxidation state and chloride in the ratio 12.
The structure of thionyl chloride showing the trigonal pyramidal geometry is shown below. The 3D chemical structure image of SULFUR DICHLORIDE is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them. USCG 1999 CAMEO Chemicals.
NOTE All the coloured pictures have java-enabled rotatable models available. Average mass 102971 Da. While drawing its Lewis structure follow the above instructions or watch the video.
Expert Answer 100 1 rating Previous question Next question. S 8 4 Cl 2 4 S 2 Cl 2. The structure is zero-dimensional and consists of eight sulfur dichloride molecules.
Sulfur Dichloride on Wikipedia. According to Lewis structure SOCl 2 has three atoms that are attached to central Sulfur that include two Chloride and Oxygen. What is the lewis structure of of2.
Do you think we have missed something about the SCl2 topic. What is molecular shape of of2. SCl2 crystallizes in the orthorhombic P2_12_12_1 space group.
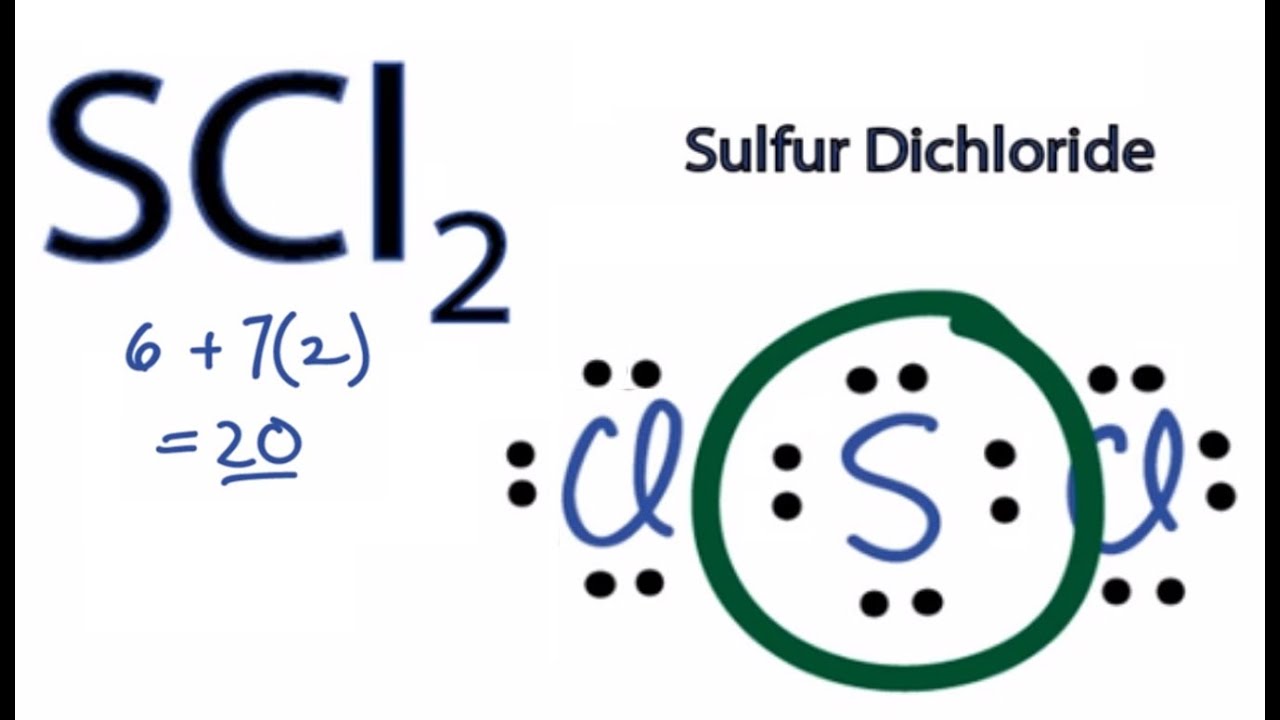
Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. SCl2 - Sulfur Dichloride. Monoisotopic mass 101909775 Da.
Monoisotopic mass 85932617 Da. What is 3-d model of of2 and scl2. Readings for this section.
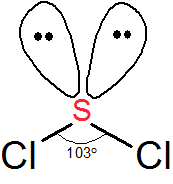
In reality however ozone has two oxygen-oxygen bonds which are equal in length and are halfway between the lengths of typical oxygen-oxygen single bonds and double bonds effectively there are two one-and-a-half bonds in. SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. These are arranged in a trigonal bipyramidal shape with a 175 F axial-Cl-F axial bond angle.
ΔH 406 kJmol. This record has not been tagged. Jan 16 2015.
Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs. This problem has been solved. Click on the image to open the page containing the java applet.
It is a beryllium molecular entity and an inorganic chloride. This record has not been tagged. The two lone pairs take equatorial positions because they demand more space than the bonds.
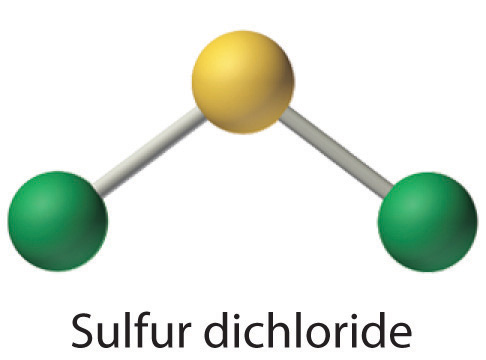
The sulfur dichloride SCl2 has bent molecular geometry. Average mass 86905 Da. The result is a T-shaped molecule.
The molecular geometry of SCl 2 is bent with asymmetric charge distribution around the central atom. It has a role as a carcinogenic agent and a genotoxin. Then draw the 3D molecular structure using VSEPR rules.
Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewiss diagram and chlorine is spaced evenly around it. Note that Sulfur is the least electronegative atom in the SCl2. There are one shorter 203 Å and one longer 206 Å SCl bond lengths.
Stabilized pure sulfur dichloride should contain minimum 98 SCl2. First draw the Lewis dot structure. ΔH 582 kJmol.
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. The SCl2 is a covalent bond whose dipole moment direction is 053D. SCl2 lewis structure contains one sulfur and two chlorine atom.
SCl 2 is produced by the chlorination of either elemental sulfur or disulfur dichloride. First draw the Lewis dot structure. The process occurs in a series of steps some of which are.
It is important to remember that Lewis structures are not meant to convey geometry so it would be wrong to assume that the molecule is linear just by looking. Therefore SCl2 is polar. Van Nostrand Reinhold Co 1987 p.
The addition of Cl 2 to S 2 Cl 2 has been proposed to proceed via a mixed valence intermediate Cl 3 S-SCl.
Lewis Structures Page 2 And Hybridizations For Common Molecules
Types Of Bonding And Lewis Structures Ppt Download

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube
Electron Geometry And Molecular Geometry 3d Sketch Chegg Com

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

Is Scl2 Polar Or Nonpolar Techiescientist

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

And Molecular Y Electron Geometry Formula Lewis Chegg Com
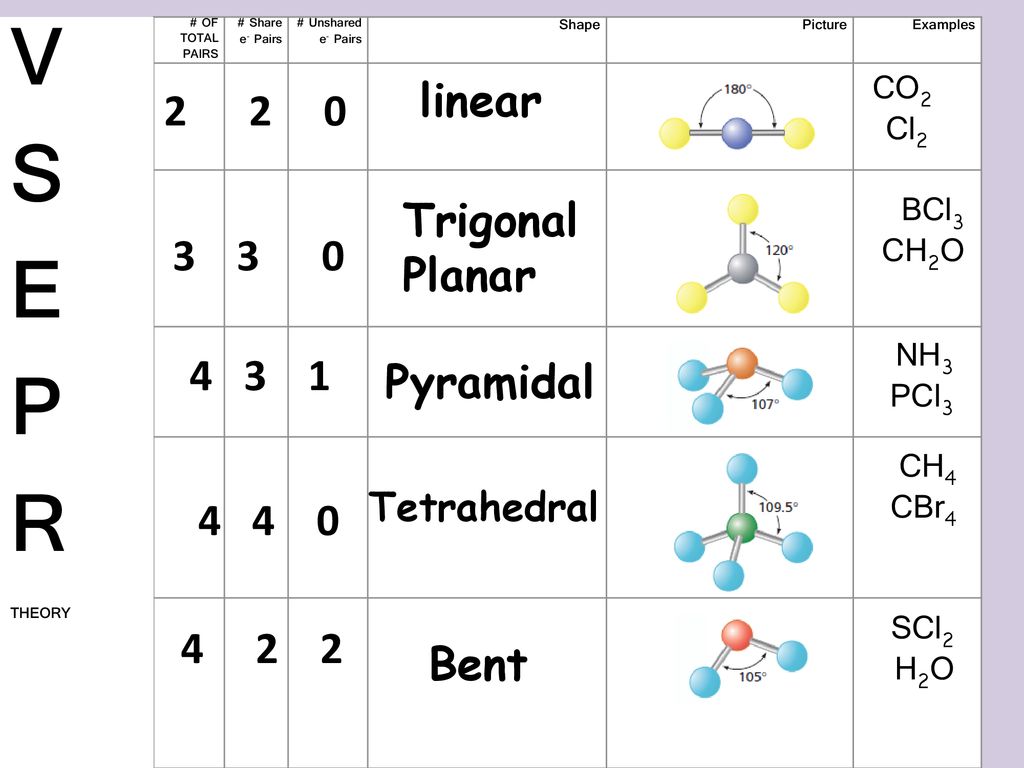
Valence Shell Electron Pair Repulsion

Sf2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
What Is The Molecular Shape Of Scl2

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep
Sulfur Dichloride Cl2s Chemspider

Sulfur Dichloride Structure Cl2s Over 100 Million Chemical Compounds Mol Instincts

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube
10 4 Writing Lewis Structures Chemistry Libretexts