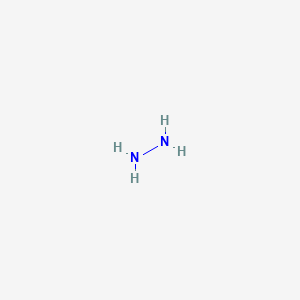
The Molecular Structure Of Hydrazine N2h4 Is Shown
I like how you made. C Each nitrogen atom shares one of its electrons with a nitrogen atom.
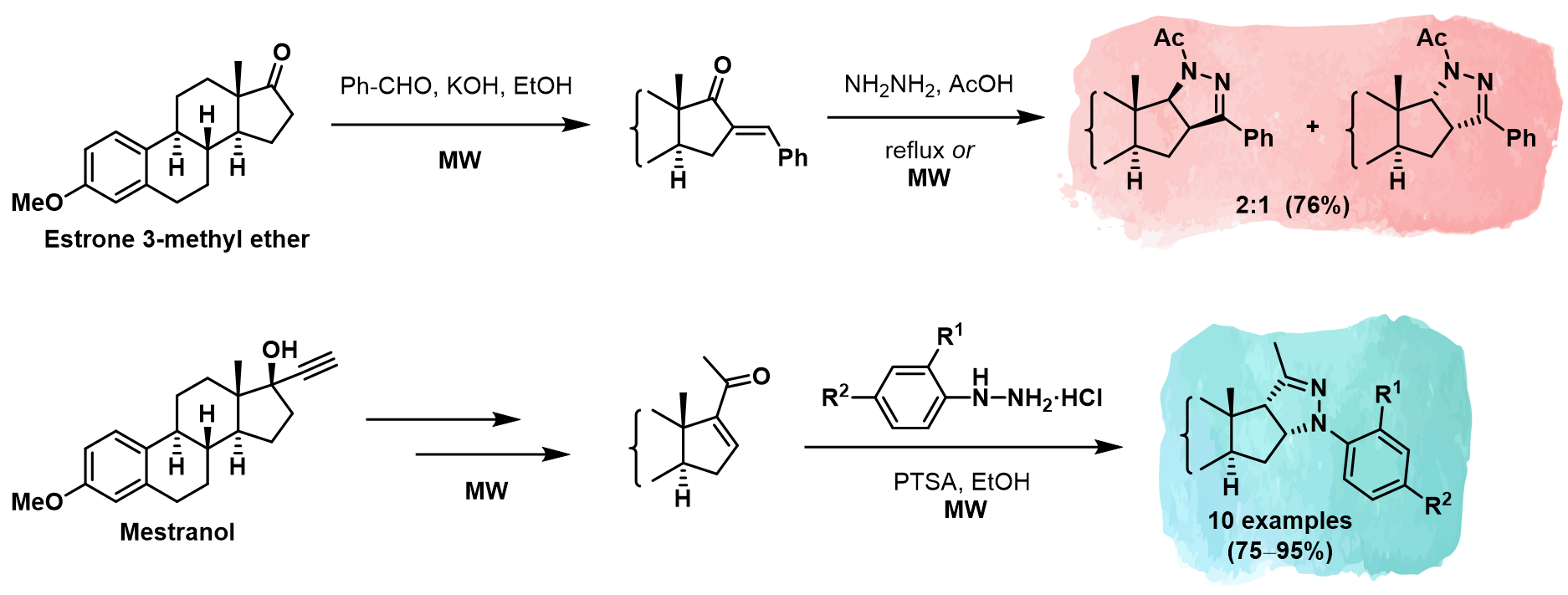
Molecules Free Full Text Microwave Assisted Stereoselective Heterocyclization To Novel Ring D Fused Arylpyrazolines In The Estrone Series Html
An atom X contains 16 protons.
The molecular structure of hydrazine n2h4 is shown. The Nitrogens originally contained five electrons and the Hydrogen only one electron. A It cannot form an ion. This is because it has an uneven distribution of electrons in the molecule.
Hydrogen H only needs two valence electrons to have a full outer shell. B Each nitrogen atom has four bonding pairs of electrons. NN H H H H Consider all the electrons in a molecule of hydrazine.
The diagram shows the structural formula of the covalent molecule hydrazine N 2 H 4. A Each nitrogen atom has a non-bonding pair of electrons. A Each nitrogen atom has a non-bonding pair of electrons.
Answer to The molecular formula for hydrazine is N2H4. B Each nitrogen atom has four bonding pairs of electrons. A What is the percent by mass of hydrogen in hydrazine to one decimal.
Hydrazine N2H4 is a good reducing agent that has been used as a component in rocket fuels. Chemistry Matter And Change Ppt Download Is n2h4 ionic or covalent why. Lets do the N2H4 Lewis structure.
Lewis structure draw. Which description of the bonding in hydrazine is not correct. Select its Lewis structure.
Hydrazine polar molecule bond line polarity dash electron sym shown unshared symmetry negative note near area covalent non. The dash model above shows that although each of the four hydrogen have an even distribution the two nitrogens do not. Which description fits the arrangement of these electrons in the molecule.
The molecular structure of hydrazine N2H4 is shown. Hydrazine N2H4 is a polar molecule. The molecular structure of hydrazine N2H4 is shown.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. N2h4 is a covalent compound because contain two nonmetals in the molecule. N2H4 is a polar molecule with London dispersion forces dipole-dipole forces and hydrogen bonding between molecules whereas C2H6 is nonpolar and only has London dispersion forces between molecules.
Which description of the bonding in hydrazine is not correct. 2 The molecular structure of hydrazine N2H4 is shown. N2H4 is straightforward with no double or triple bonds.
Solution for Draw the Lewis structure of C₂H₄Cl₂ both Cl atoms on one C atom and then determine if the molecule is polar or nonpolar. Lewis dot n2 diagram structure. Also the arrows showing the lower electronegativity to the higher negativity are correct as the Nitrogen has a higher level than the Hydrogen.
It takes more energy to overcome the stronger IMFs in hydrazine. Hydrazine lewis structure polar molecule uneven intermolecular forces because electrons polarity dash attraction. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
Molecular geometry bond angle angles. Nitrogen has five valence electrons. Hydrazine N2H4 is a good reducing agent that has been.
You have two Nitrogens. The drawing of the Hydrazine molecule looks correct with the proper angles for a pyramidal structure on both ends. A H N N H H H B H N N H H H C H N N.
You showed all three forces of attraction which take place between N2H4 and another N2H4 molecule. A Each nitrogen atom has a non-bonding pair of electrons. The name of the molecular compound n2h4 is hydrazine.
How to draw lewis structure for N2H4Hydrazine Lewis structure of N2H4 is made up of two nitrogen and four hydrogens having two lone pairs on the nitrogen atomsone lone pair on each nitrogen and contain a total of 10 shared electrons. Which statement about X is correct. B Each nitrogen atom has four bonding pairs of electrons.
Which description of the bonding in hydrazine is not correct. C Each nitrogen atom shares one of its electrons with a nitrogen atom.

Molecules Free Full Text Current Synthetic Routes To Peptidyl Mono Fluoromethyl Ketones Fmks And Their Applications Html

Molecules Free Full Text An Alternative Approach For The Synthesis Of Sulfoquinovosyldiacylglycerol Html

Hydrazine An Overview Sciencedirect Topics
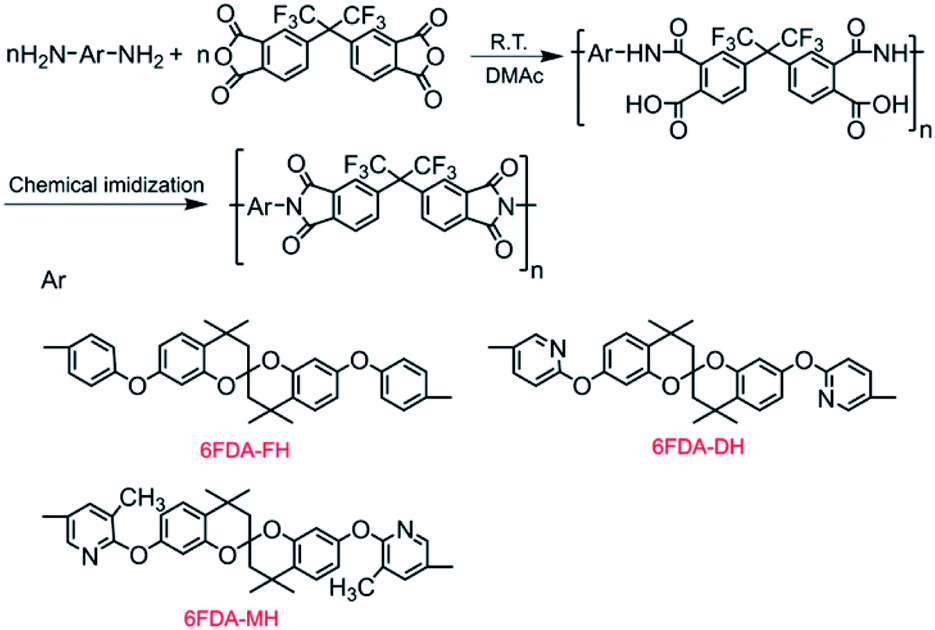
The Spirobichroman Based Polyimides With Different Side Groups From Structure Property Relationships To Chain Packing And Gas Transport Performance Rsc Advances Rsc Publishing Doi 10 1039 D0ra10113c

Chemistry 2 Atoms Molecules And Ions Youtube Molecules Molecular Chemistry
Hydrazine Formula N2h4 Or H4n2 Is A Highly Reactive Base And Reducing Agent Used In Many Industrial And Medical Applications 3d Illustration The Molecule Is Represented In Different Structures

Assembly Of Hydrazine Configurations 3 A 3 B And 3 C For Three Download Scientific Diagram

Molecules Free Full Text An Alternative Approach For The Synthesis Of Sulfoquinovosyldiacylglycerol Html
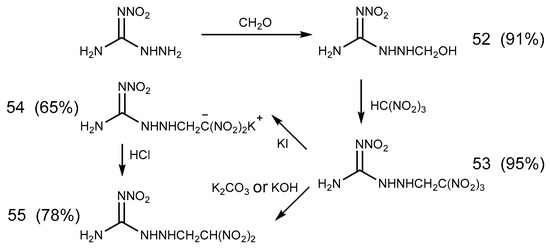
Molecules Free Full Text A Review On The Reactivity Of 1 Amino 2 Nitroguanidine Anq Html

Hydrazine Formation Via Coupling Of A Nickel Iii Nh2 Radical Gu 2021 Angewandte Chemie Wiley Online Library

Molecules Free Full Text An Alternative Approach For The Synthesis Of Sulfoquinovosyldiacylglycerol Html

Molecules Free Full Text Microwave Assisted Stereoselective Heterocyclization To Novel Ring D Fused Arylpyrazolines In The Estrone Series Html
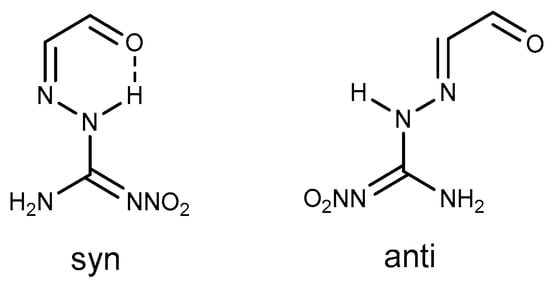
Molecules Free Full Text A Review On The Reactivity Of 1 Amino 2 Nitroguanidine Anq Html

Hydrazine Acetate 97 7335 65 1

Welcome To Quilava S Blog Chemistry Education Structural Formula Nurse Drawing