Vsepr Structure Of Sif4
Sulfur tetrafluoride has 5 regions of electron density around the central sulfur atom 4 bonds and one lone pair. Predict the geometry using the VSEPR model and draw the Lewis structure the of the following ions.
Point group T d.

Vsepr structure of sif4. SiF 4 - Silicon tetrafluoride. The Xenon atom has 4 bonding pairs of electrons and 2 lone non-bonding pairs of electro. See the answer See the answer See the answer done loading.
According to the VSEPR theory every atom in a molecule will adopt geometry such that the repulsion between the valence electrons in the atoms is minimal. S has 4 valence electrons plus 1 for each Si-F single bond. Silicon tetrafluoride SiF4 or F4Si CID 24556 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Principles of the VSEPR method. Count its valence electrons 3. Divide the total of these by 2 to find the total number of electron pairs.
1 NSF 3 SiF 4 POF 3. These are arranged in a trigonal bipyramidal shape with 102 F-S-F bond angles between the equatorial fluorine atoms and 173 between the axial fluorine atoms. Pairs of electrons in the valence shell of a central atom of a molecule repel each other and take up positions as far apart as possible.
The valence electrons that participate in forming bonds are called bonding pairs of electrons whereas the electrons that do not participate or form any. VSEPR THEORY - BOND ANGLES - NSF 3 SiF 4 POF 3 3 The correct order of FMF bond angles where M is the central atom in NSF 3 SiF 4 and POF 3 is. Add or subtract electrons for charge see Top Tip 5.
It is a theory that allows us to predict the 3D arrangement of the atoms of a molecule based on the repulsion between negatively charged. What do valence electrons represent in a Lewis structure. VSEPR stands for Valence Shell Electron Pair Repulsion.
One attribute of VSEPR is that with the ability to predict the shape of a molecule for a compound comes the ability to predict some of the physical and chemical properties of that compound. Total 8 electrons four pairs. Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP.
The melting and boiling point of silicon tetrafluoride is -950 C and -903 C and hence it exists as a gas at room temperature. The formal definition that is the basis for VSEPR is as follows. Powell idea and developped the theory of the valence shell electron pair repulsion VSEPR.
It is named tetrafluorosilane or silicon tetrafluoride. A SiF4 b SF. A SiF4 b SF.
Predict the geometry using the VSEPR model and draw the Lewis structure the of the following ions. 2 SiF 4 NSF 3 POF 3. SiF4 is tetrahedral so that the individual dipoles on the Si-F bonds cancel and the molecule has no dipole moment.
Hence the bond angle is maximum ie. In the VSEPR model the molecule or polyatomic ion is given an AX m E n designation where A is the central atom X is a bonded atom E is a nonbonding valence electron group usually a lone pair of electrons and m and n are integers. The bond angle is least affected in case of SiF4 since all the Si-F bonds are single bonds which exert less repulsion on other bond pairs.
And the electron pairs prefer to occupy maximum distance from each other. This problem has been solved. 4 SiF 4 POF 3 NSF 3.
Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. Add one electron for each bonding atom 4. Jmol_Canvas2D Jmol jmolApplet0 x.
Identify the central atom 2. 3 NSF 3 POF 3 SiF 4. Structure tetrahedral class AX 4.
The VSEPR theory varies for molecules with the presence of lone pairs and multiple bonds around the central atom. The bonds formed between two atoms are depicted using lines whereas the valence electrons not forming any bonds are shown by dots. Origin In 1957 the canadian chemist RJ Gillespie university Mc Master Hamilton Ontario took back british N.
In the Lewis symbol for an atom the chemical symbol of the element as found on the periodic table is written and the valence electrons are represented as dots surrounding it. SIF4 is a covalent compound which consists of silicon and fluorine atoms. Mark me as brainlist if you get this.
This method prolongs in the geometrical shape aspect the description of the chemical bond by G. Electron pairs gives base shape Octahedral VSEPR base shape for 6 e-pairs Refcode. Xenon tetrafluoride XeF4 is a square planar non-polar molecule.
SiF4 Lewis Structure Molecular Geometry Hybridization and Polarity.

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Molecular Geometry Bond Angles Electron Geometry Silicon Tetrafluoride Youtube

Icl4 Lewis Structure Tetrachloroiodide Ion Youtube

Scl2 Molecular Geometry Sulfur Dichloride Youtube

Krf4 Lewis Structure How To Draw The Lewis Structure For Krf4 Krypton Tetrafluoride Youtube

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Chem Molecular Shape Molecular Geometry Scientific Tutor

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State

Bro3 Lewis Structure Bromate Ion Youtube

Sf4 Molecular Geometry Shape Youtube

Science Coverage Is Asf5 Polar Or Nonpolar In 2021 Molecular Geometry Molar Mass Octet Rule

Bro3 Lewis Structure Bromate Ion Youtube

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Science Coverage Is Hbr Polar Or Nonpolar In 2021 Electron Affinity Solubility Polar

Science Coverage Is Hf Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

Science Coverage Is No2 Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Cf4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
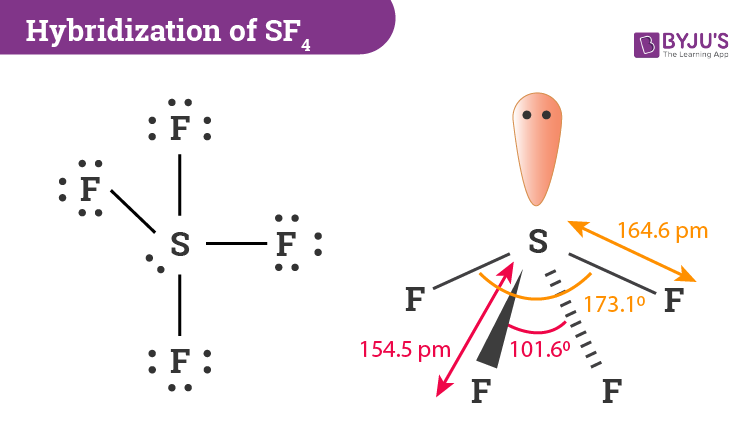
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride
