Hcn Lewis Structure Vsepr
BF3 Lewis Structure VSEPRSketch Polarity Molecular shape B. Ai only Bi and iv Cii and iv Di and iii Ei and v 2In the resonance form of ozone shown below the formal charge on the central oxygen atom.
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape
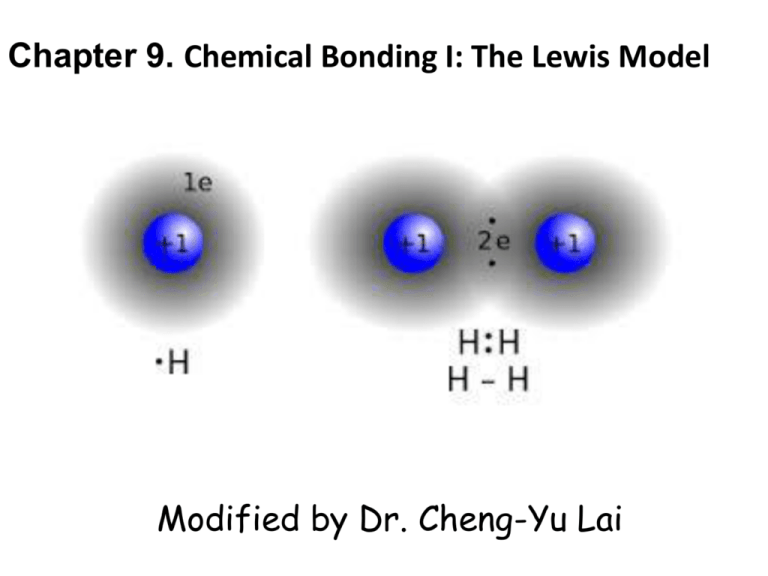
The electronic structure of molecules can be illustrated by Lewis structures which can be used to and properties such as geometry bond orders bond lengths relative bond energies and dipoles.
Hcn lewis structure vsepr. The Lewis structures including resonance forms are. Therefore the molecular shape is. HCN VSEPR Lewis Structure images similar and related articles aggregated throughout the Internet.
1 x triple bond. Lewis structures of H 2 O and SO 2. The number of electron groups around the central P atom is 3 this is true in any of the resonance forms The distribution of electron groups is trigonal planar.
Lewis structure of HCN molecule. Lewis electron structures give no information about molecular geometry the arrangement of bonded atoms in a molecule or polyatomic ion which is crucial to understanding the chemistry of a molecule. IPCl3 iiCH2Cl2 iiiHCN ivC2H4 vNH3 1In the Lewis structures of _____ the central atom has one lone pair of electrons.
Learn to draw the Lewis structure of HCN understand molecular geometry shape polarity about the same by reading this article. There are both electron domain and molecular geometries which may sometimes. VSEPR theory states that the valence electron pairs at the central atom will arrange themselves in a certain geometry.
Lewis structures are two-dimensional representations of molecules. This causes a deviation from ideal geometry an HCH bond angle of 1165 rather than 120. Make sure you put the correct atom at the center of the HCN molecule.
All electron groups bond terminal atoms. Determining the hybridization can be difficult. However most molecules are three- dimensional.
Lewis Structures Hyperconjugation-Octet Rule Binding of n atoms to central atom using fewer than n electron pairs on the central atom SF 6 Total Valence electrons VE 6 7 7 7 7 77 48 Number of Bonding electrons BE 4 Bonding pairs Number of lone pair electrons LP 48 8 40 20 Lone pairs All bonds are equal F F F 2 -1 Plus other structures S F F -1 F F F F 2 -1 S F F -1 F. Valence Shell Electron Pair Repulsion VSEPR theory along with Lewis structures can be used to predict. For the HCN Lewis structure.
HCN Lewis Structure VSEPR Sketch PolarityMolecular shape Viewing Notes. The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. Gillespie at McMaster University in Hamilton developed the VSEPR Theory to explain the shapes of molecules.
He was one of Mrs. Be sure that you dont use more than the ten valence electrons available. A molecules shape depends on the number of bonded pairs BP and lone pairs LP around the central atom.
Now you can see that Nitrogen has eight valence electrons and Carbon has six. According to VSEPR theory this valence bond configuration corresponds to a linear molecular geometry around the central atom as described by the Lewis structure. Molecule of valence e-Lewis Structure NO 3-5 36 1 24e- The Valence Shell Electron Pair Repulsion Theory VSEPR states the electron pairs surrounding an atom tend to repel each other.
In Hydrogen cyanide HCN lewis structure carbon forms one single bond with the hydrogen atom and a triple bond with the nitrogen atom.
Chemical Bonding An Introduction 1 Chemical Bond Ionic
Hcn Lewis Structure And Molecular Geometry Step By Step Youtube
Draw The Lewis Structure For Krf2 The Cen Clutch Prep
4 1 Molecular Shape Chemistry Libretexts
Vsepr For 2 Electron Clouds Video Vsepr Khan Academy
Hcn Lewis Structure Molecular Geometry What S Insight
Draw The Lewis Structure Of Clbr3 Showing Clutch Prep
Molecular Structure And Polarity Chem 1305 Introductory Chemistry
Vsepr Theory And Molecular Geometry Bf3 Ch2o Hcn Becl2 Ch2cl2 Socl2 So2 Youtube
How To Draw Lewis Dot Structures Easiest Method Chad S Prep
Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube
Hcn Molecular Geometry Youtube
6 3 Molecular Shape Introductory Chemistry
Hcn Lewis Structure Molecular Geometry Shape And Polarity

