Lewis Structure So2 Following Octet Rule
Octet Rule and Lewis Structure for Electron. Relevant Posts - Appropriate Videos Lewis.

So2 Lewis Structure Valence Electrons Formal Charge Polar Or Nonpolar
S does not follow the octet rule.

Lewis structure so2 following octet rule. Connect the atoms with single bonds. Where n in this case is 3. See full answer below.
From an experimental view using x-ray crystallography or someth. It can hold more than 8 electrons. SO2 contains S 6 2 O 6 18 valence electrons.
If this is the case sulfur will. Most structuresespecially those containing second row elementsobey the octet rule in which every atom except H is surrounded by eight electrons. S does not follow the octet rule.
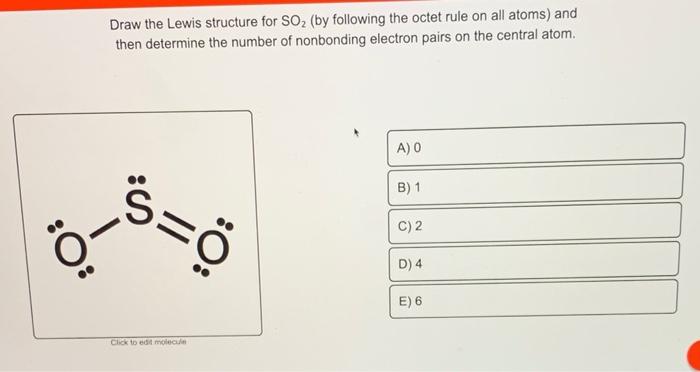
Draw the Lewis structure of SO₂ by following the octet rule on all atoms and then determine the ideal bonding angles of the central atom. Resonance structures of SO 3 2-are drawn after drawing the lewis structure. Lewis Dot of Sulfur Dioxide SO2.
Sulfur is at 4. It represents forms of Chemical equation with the help of structured atomsSO2Sulfur Dioxide Molecular Geometry Lewis StructureThe Sulfur Dioxide which is also known as Sulphur. Oxygen2 does not share instead is donated 2e by S ie.
NOT IN THIS MOLECULE. Simply we can see in the structure or SO2 that oxygen1 has 8 e in outermost shell as 2 it shares with S. A dative bond is formed and so it also get 8 e in outermost shell.
S does not follow the octet rule. It can hold more than 8 electrons. These are exceptions to the octet rule and the particular elem.
A step-by-step explanation of how to draw the SO2 Lewis Structure Sulfur Dioxide Note. Therefore P 6n 2 V 6 3 2 - 18 2 Therefore there is a double bond in the molecule. Where V 6 6 6 18 V is the number of valent electrons of the molecule.
NOT IN THIS MOLECULE. Lewis Dot of Sulfur Dioxide. 70 More Lewis Dot Structures.
S does not follow the octet rule. Regarding this does so2 obey octet rule. The Lewis dot structure of SO2 or sulfur dioxide has a central atom of sulfur that violates the octet rule.
It causes a repulsion of electron pairs to form the 120-degree angleBy analyzing the Lewis structure of SO2 we can see that the SO2 is asymmetrical because it contains a region with different sharing. Calculate electrons in π multiple bonds by formula 1. Draw The Lewis Structure Of So2 By Following The Octet Rule On All Atoms Unit 4 Review Objectives 11 19 flashcards Quizlet The Correct Lewis Structure of Sulfur Dioxide YouTube SO3 2 Lewis Structure How to Draw the Lewis Structure SOLVEDThese species do not obey the octet rule.
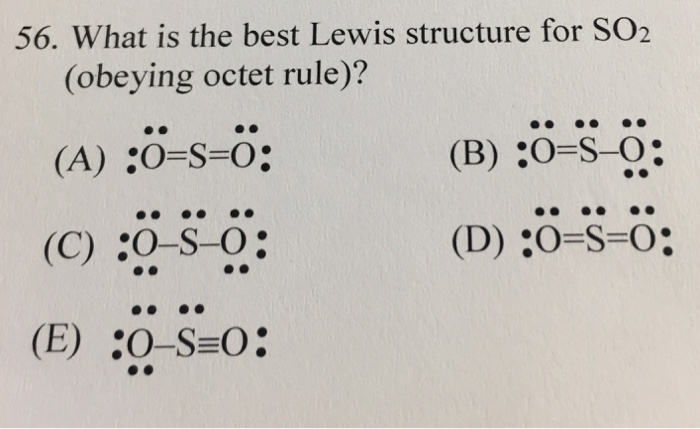
So2 lewis structure octet rule Step 1. O2 Lewis Structure Octet Rule Lewis Dot Structure Example Octet Rule Exception Lewis Structure of SO2 sulfur dioxide YouTube Molecular structure and bonding Part 11. Draw a Lewis structure for SO2 in which all atoms obey the octet rule.
In this case and in many Lewis structures we will draw we leave off the implied lone pairs around the peripheral atoms. Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates. Sulfur having valence electrons in the 3rd energy level would also have access to the 3d sublevel thus allowing for more than 8 electrons.
Now coming to S it has 6e of its own and 2 e which it shares with oxygen1 this it also has total of 8e. 70 More Lewis Dot Structures. S does not follow the octet rule.
So all of them follow octet rule. It can hold more than 8 electrons. It is produced from the burning of fossil fuels coal and oil.
Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates. S does not follow the octet rule. What is so2 made up of.
Lewis Dot of Sulfur Dioxide. Sulfur having valence electrons in the 3rd energy level would also have access to the 3d sublevel thus allowing for more than 8 electrons. 2 atoms 2 3 lone pairs 12 more electrons.
Draw lewis structure so2 atoms obey octet rule show formal charges. The remaining 2 electrons go on the central atom as a lone pair. For sulfur dioxide or SO2 S O 2 to obey the octet rule its structure should have the O and S atoms surrounded by eight electrons may.
Remember Sulfur is in Period 3 and can hold more than 8 valence electrons. This short video gives three elements that can form stable compounds with incomplete octets. There are three resonance structures SO2 Sulfur dioxide.
Lone pairs unpaired electrons and single double or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure. Sulfur dioxide SO2 is a colorless gas or liquid with a strong choking odor. 70 More Lewis Dot Structures.
With one sulfur atom we place it at the center since it is less electronegative than oxygen. Try calculating the number of bonds in each of these molecules using the N-V method and fill in the lone pairs that are not explicitly drawn in. Shown by dots In SO2 Lewis structurethere are two double bonds that are going from the atom sulphur to oxygensIn SO2 Lewis structurethere are totally five lone pairs of electronsThe each oxygen has two lone pairs of electrons and the sulphur atom has one lone pair of electronsIn SO2 Lewis structurethe oxygen atoms follow the octet rule.
Exceptions to the octet rule occur for odd-electron molecules free radicals electron. Octet structures of the Lewis acid-base adduct NH 3 BF 3 the hydronium ion H 3 O and the sulfate anion SO 4 2-are shown below.

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

17 A Valid Lewis Structure Of A So2 B Cf Cannot Chegg Com

Get Answer Draw A Lewis Structure For So2 In Which All Atoms Obey The Octet Transtutors

So2 Molecular Geometry Hybridization Lewis Structure Mo Diagram

Draw A Lewis Structure For So2 In Which All Atoms Obey The Octet Rule Show Formal Charges Do Not Brainly Com
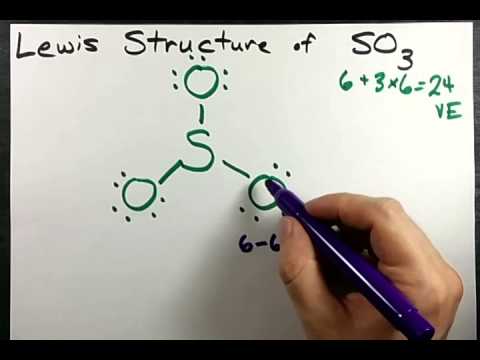
Lewis Structure Of So3 Sulfur Trioxide Youtube

What Is The Correct Lewis Structure For So2

Lewis Structure Of So4 2 Sulfate Correct Youtube

Sulfor Dioxide Lewis Dot Structure For So2 Video Khan Academy

Lewis Dot Structure General Chemistry Quiz Docsity
Draw A Lewis Structure For So2 In Which All Atoms Chegg Com

The Correct Lewis Structure Of Sulfur Dioxide Youtube

So2 Lewis Structure All Atoms Obey Octet Rule

Draw A Lewis Structure For So2 In Which Al Clutch Prep
Draw The Lewis Structure For So2 By Following The Chegg Com

So2 Lewis Structure Sulfur Dioxide Youtube

Lewis Dot Of Sulfur Dioxide So2
What Is The Best Lewis Structure For So 2 Obeying Chegg Com
Does So2 Obey The Octet Rule Quora