C2h6 Lewis Structure Valence Electrons
The C2H6 Lewis structure. On the periodic table Carbon is in group 4 or 14 so it has 4 valence electrons.

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar
CO Lewis Structure In CO Lewis.
C2h6 lewis structure valence electrons. The first step in drawing the Lewis dot structure for ethane C_2H_6 is to determine how many valence electrons are available for the molecule. Hence Ethene is planar. In the case of carbon we have four valence electrons each.
Drawing the Lewis Structure for C 2 H 2 Ethyne or Acetylene. Both use all 20 valence. Each C atom forms three covalent bonds with three H atoms with one aditional covalent bond being formed between the two C atoms.
Draw the Lewis structures of C2H6 C2H4 and C2H2Draw the molecules by placing atoms on the grid and connecting them with bonds. To count the valence electron in ICl2- molecule look at the periodic group of iodine and chlorine atom in the periodic table. Hydrogen is the first element in the periodic table therefore it has only one valence electron.
Use information from step 4 and 5 to draw the lewis structure. A lewis electron dot structure shows how the atoms of a molecule or an ion share their outermost electrons or valence electrons to form covalent bonds with each other. CO Lewis structurecarbon monoxide electron dot structure is that type of structure where we show the total ten valence electrons of CO as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots.
The C2H6 Lewis structure has a total of 14 valence electrons. C 6 H 6 has a total of 18 valence electrons. Count total valence electron in ICl2-As the lewis diagram is all about filling the valence electron around the atoms within a molecule hence find the total valence electron that is available for drawing the lewis structure of ICl2-.
Calculate the total valence electrons in the molecule. B What is the hybridization of the carbon atoms in each. Calculate the total valence electrons in themolecule.
Lewis dot structure of C2H6 Alternatively a dot method can be used to draw the lewis structure. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. Use information from step 4 and 5 to draw the lewis structure.
After determining how many valence electrons there are in C2H6 place them around the central atom to complete the octets. To draw the Lewis structure for C2H4 the total number of valence electrons must be known. Lewis dot structure of C 2 H 6.
A Draw Lewis structures for ethane C2H6 ethylene C2H4 and acetylene. Since both iodine and chlorine. This means that the Lewis dot structure for C2H 6 must account for 14 valence electrons either through bonding between atoms or through lone pairs.
Hydrogen H atoms always go on the outside of a Lewis structure. Together we can come to a right answer. Each carbon in the Ethylene Lewis structure has Sp² hybridization and with two hydrogens it makes the structure look like a triangular planar which is two-dimensional.
Alternatively a dot method can be used to draw the lewis structure. We have 6 multiply that by 6 for a total of 14 valence electrons to work with. CO Lewis Structure Answer.
Electron Dot Diagram For C2h6. Why C2H4 lewis structure is planar and C2H6 is non-planar. Put two carbons in the center and arrange hydrogena toms on the sidesArrange electrons until both carbons get 8 electrons.
Alternative Representations of Methane. So the two C atoms are placed in the center of the molecule. B What is the hybridization of the carbon atoms in each molecule.
14 C2H6 Lewis Structure. Submit Your â Here a 25 Å resolution structure of four ZF domains of Snail1 complexed with importin β is presented. The total number of valence electrons in one molecule of C2H4.
Lewis Electron Dot Structures. Whereas C2H6 has Sp³ hybridization and its geometry is trigonal bipyramidal which is 3-dimensional. After determining how many valence electrons there are in C2H6 place them around the central atom to complete the octets.
The first step in drawing the Lewis dot structure for ethane C2H6 is to determine how many valence electrons are available for the molecule. They follow the duet rule 2 electrons.

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
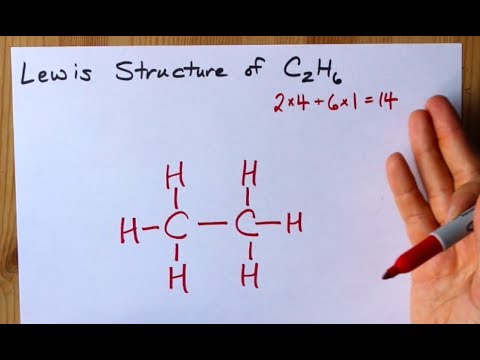
Lewis Structure Of C2h6 Ethane Youtube

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
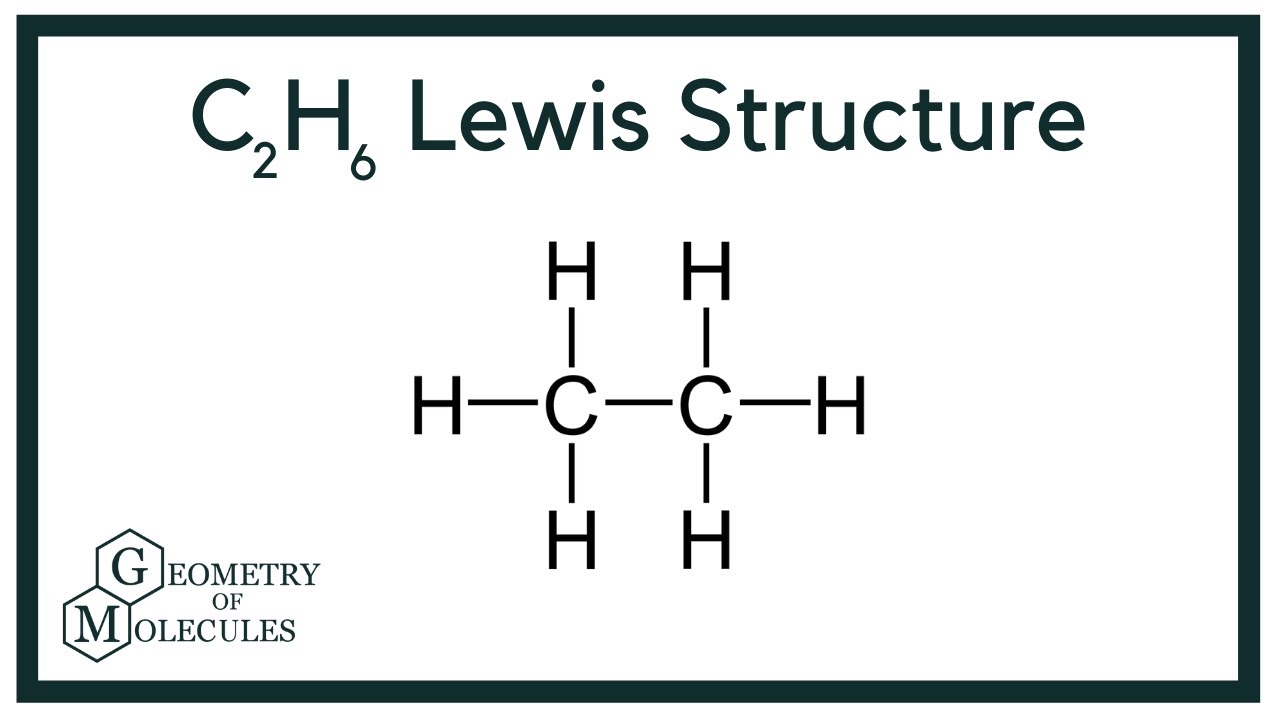
C2h6 Lewis Structure Ethane Youtube

Figura 1 Notacao De Lewis Para Os Atomos Neutros De Hidrogenio E Carbono E Para As Moleculas De Agua Etileno Eteno Chemical Bond Chemistry Notes Chemistry
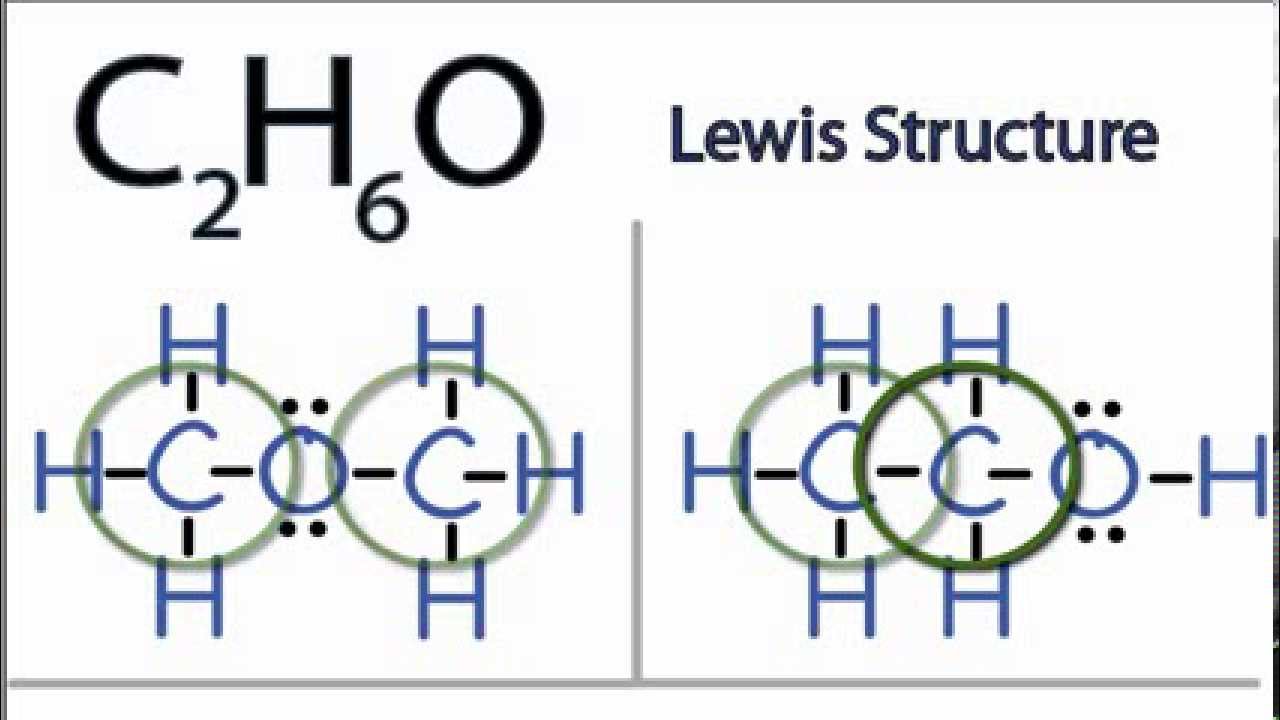
C2h6o Lewis Structure How To Draw The Lewis Structure For C2h6o Youtube

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
Lewis Structure Of C2h6 Biochemhelp

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium
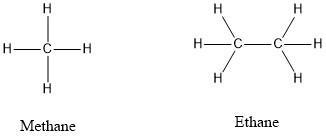
Draw The Lewis Structure For Methane Ch4 And Ethane C2h6 In The Box Below Then Predict Which Would Have The Higher Boiling
