Draw Lewis Structure For Co2
After finishing the lewis structure of CO 3 2- there should be a -2 charge and it should be stabile structure. The tension of the unhappy oxygen really wanting the electrons destabilizes this particular resonance structure.
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2
6 valence With 10 electrons one might predict this structure.

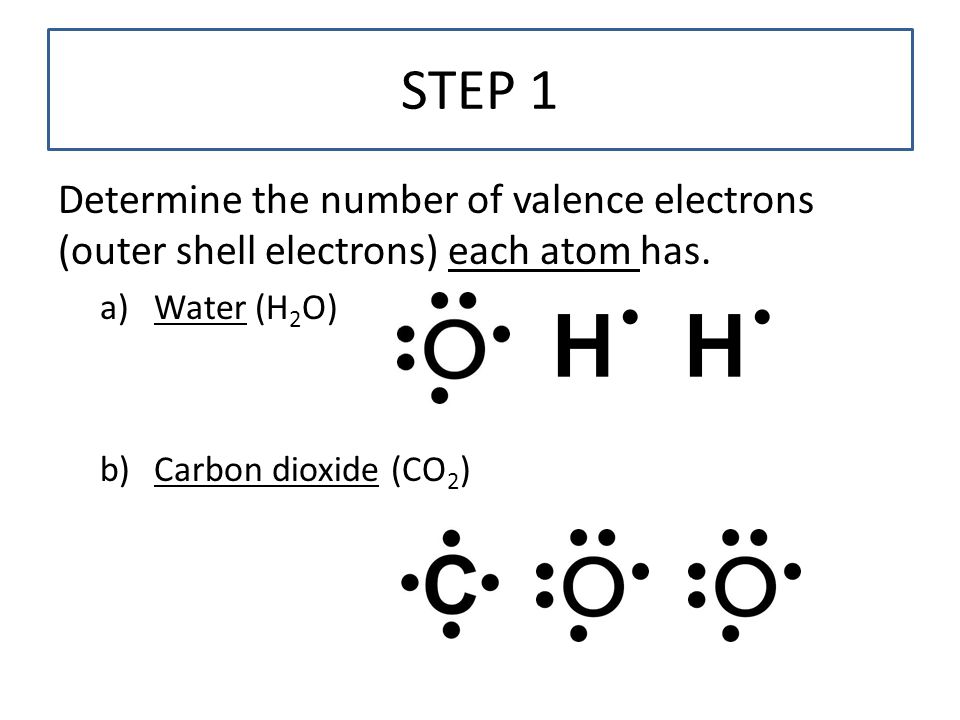
Draw lewis structure for co2. To know the lewis structure of CO2 one should first understand what precisely the Lewis structure is. These valence electrons are represented by drawing dots around the individual atoms hence the Lewis dot structure. Drawing lines represent the bonds formed in the.
A Lewis structure is a graphic representation of the electron distribution around atoms. Homework solution attached Purchase this answer to view it This homework is solved by this writer. What Is Co2 Lewis Structure And How To Draw It.
Charge valence electrons - owned electrons C. So we have 12 plus 4 16 total valence electrons. Draw Lewis Structure For Co2 co2 lewis structure dot dioxide carbon electron diagram electrons resonance pairs structures bonds many covalent lone valence oxygen ionic double dioxide lewis carbon co2 structure molecular dot draw structures chemical chemistry monoxide lewis co2 structure carbon dioxide draw centre oxygens either put going then side co2 dot lewis diagram structure.
The Lewis structure of any molecule is the representation of the molecule in the pictorial form in which the bonding between the atoms of the molecule is shown by following the octet rule or duet. Include lone pairs and formal charges. How To Draw Lewis Dot Structure For Co2 lewis molecular co2 structure structures dioxide dot carbon draw chemistry co2 lewis structure dot dioxide carbon electron diagram resonance pairs electrons structures o2 bonds draw covalent many lone valence double co2 lewis structure carbon dioxide draw centre dioxide carbon lewis structure diagram draw dot wiring.
The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. On the periodic table Carbon is in group 4 or 14 sometimes. Because molecule is symmetric around carbon atom and two oxygen atoms are located in a same line CO 2 become a non-polar compound.
Draw the Lewis structure of CO. Clients rating on draw the lewis structure for co. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule.
Lets draw the structure. I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. Lewis structure of carbonate ion is drawn in this tutorial step by step.
Include lone pairs and formal charges. And then Oxygen is in group 6 or 16. That means its going to go at the center.
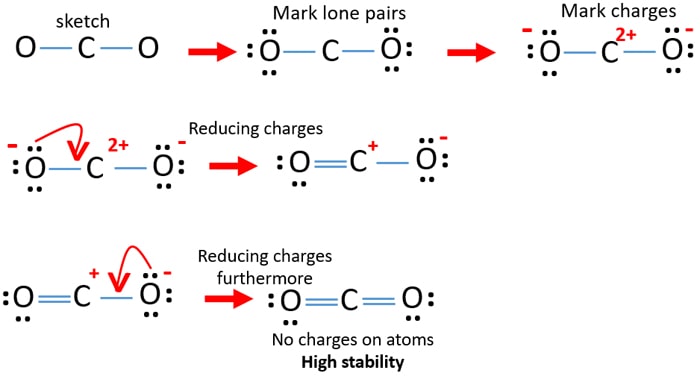
When you drew the lewis structure of CO 2 its shape is linear. Please dont forget the formal charges. Here in this post we described step by.
After determining how many valence electrons there are in CO place them around the central atom. FC 2 But that is not likely correct. Carbon is the least electronegative.
In order to complete the octets for all of the atoms in the structure you will need to form two double bonds. Draw the Lewis dot structure of CO2 molecule. FC -2 O.
This problem has been solved. I also go over hybridization shape and bond angles. Drawing CO2 Lewis Structure is very easy to by using the following method.
So well put the Carbon right here. Formal charges can be defined simply by. Answer to Draw the Lewis structure for CO.
So lets multiply that together there. See the answer See the answer See the answer done loading. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons.
You can always ask and chat with this writer about your homework needs. Were going to do the Lewis structure for CO2 Carbon dioxide. Lewis Structure for CO 3 2- Carbonate ion.
Carbon is less electronegative than oxygen so it wont be happy with having more electrons than oxygen. You will learn about these facts in this tutorial. United Kingdom 48 7161 Orders Completed.
The carbon dioxide chemical formula is CO2. But remenber that carbon atoms and oxygen atoms have. Draw the Lewis structure of CO.
A Lewis structure also helps to make a prediction about the geometry of a molecule. But we have two of them. Total valence electrons concept is used to draw the lewis structure of CO 3 2-.
Lewis Dot Structures Ppt Video Online Download

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
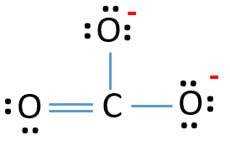
Lewis Structure For Co32 Carbonate Ion

Co2 Lewis Structure Carbon Dioxide Youtube

Lewis Structure For Co Carbon Monoxide Youtube

Co2 Lewis Structure Easy Hard Science

Co2 Lewis Structure And Molecular Geometry What S Insight
Co2 Lewis Structure Molecular Geometry And Hybridization

Draw The Electron Dot Structure Of Carbon Dioxide Co2 Brainly In

Makethebrainhappy The Lewis Dot Structure For Co2
Co2 Carbon Dioxide Lewis Structure And Shape

Lewis Electron Dot Structures Detailed Explanation With Examples Videos
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Video Dailymotion

Co2 Lewis Structure Carbon Dioxide Youtube

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube
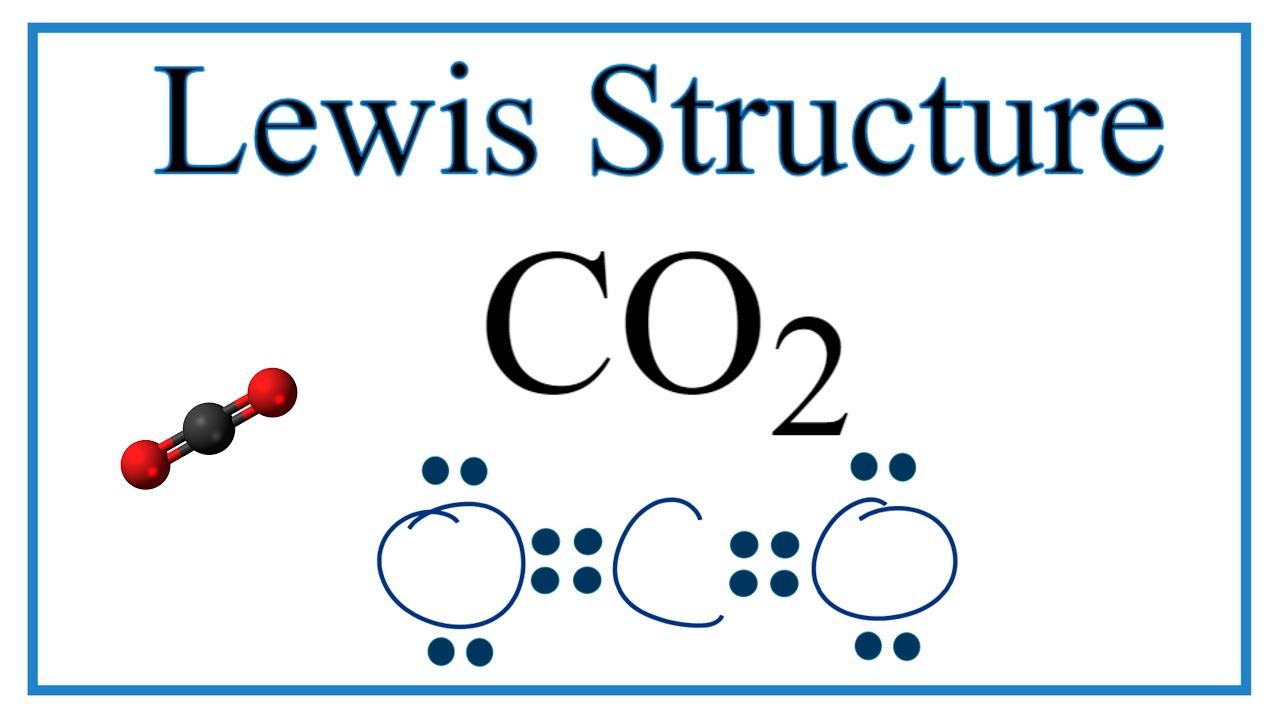
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization

Co2 Lewis Structure Easy Hard Science