Draw The Lewis Structure Of Xef2
We must first draw the Lewis structure for XeF₂. Welcome to Sarthaks eConnect.

Molecular Geometry Report Sheet High School Chemistry Core Concept Cheat Sheet 17 Molecular Geometry Vsepr Theory Chemistry Basics
Is XeF2 trigonal bipyramidal.

Draw the lewis structure of xef2. So for this compound XeF2 there is one molecule of Xenon and two molecules of Fluorine. Draw the structure of i XeF2 ii H4P2O7. Hydrogen H only needs two valence electrons to have a full outer shell.
Total number of valence electrons No. Asked Apr 20 2019 in Chemistry by Bhawna 686k points cbse. Draw Lewis structures for CCl 4 and C 2Cl 4.
In XeF2 there are 5 pairs of electrons around Xe and thus its geometry is. A single molecule of Xenon has eight electrons and a Fluorine molecule has seven valence electrons. They are the three lone pairs and the two Xe-F bonds.
Drawing the Lewis Structure for XeF 2. Draw lewis structure of H2O. Step 2 The next step asks us to distribute the valence electrons in the molecule all around the central atom.
Of valence electrons for Fluorine. Considering the central atom in XeF2 i Identify the total number of electron pair groups 1 mark. Xenon Xe can have more than 8 valence electrons in your Lewis structure.
Drawing the Lewis Structure for XeF 2. Draw Lewis structure for ONH2. Drawing Lewis structure of XeF4.
Trn it Xe is sp 3 d 2-hybridised but its shape is square planar due to involvement of VSEPR theoryie due to presence of two free pair of electrons geometry of XeF 4 is distorted. Draw the best Lewis structure for ClO4-. AsF3 CH3 BrF3 ClO3.
E Number of lone pairs on central atom. Remember that Xenon can have more than 8 valence electrons. I also go over hybridization shape and bond angle.
The Lewis structure for XeF 2 requires you to place more than 8 valence electrons on Xe. Draw Lewis structure of XeF2. I quickly take you through how to draw the Lewis Structure of XeF2 Xenon DiFluoride.
100 7 ratings XeF2. Explain why the carbon atoms in the two molecules have different shapes. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule.
This is the best answer based on feedback and ratings. Draw lewis structure of HCN. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for XeO 2 F 2.
ANumber of central atoms. There are a total of 22 valence electrons in the Lewis structure for XeF2. OTHER SETS BY THIS CREATOR.
In it Xe is sp 3 d-hybridised but its shape is linear due to involvement of VSEPR theory. Xenon Xe can have more than 8 valence electrons in your Lewis structure. Draw lewis structure of XeF2.
Iii State the molecular shape 1 mark iv What is the hybridization or give the hybrid orbital type 2 mark. A unique platform where students can interact with teachersexpertsstudents to get solutions to their queries. Draw lewis structure of SF4.
XNumber of surrounding atoms. Ii Identify the number of lone pairs 1 mark. Of valence electrons for Xenon No.
Ib chemistry 4-2 covalent bonding. How to find the shape of XeF 2 using VSEPR theory. Apply VSEPR notation A X E.
Draw lewis structure of BrF5 and XeF2 using VSPER theory. 6 rows Drawing the Lewis Structure for XeF 2. Give the molecular shape around each carbon atom.
The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. Ie due to presence of three free pair of electrons geometry of XeF 2 is distorted from trigonal bipyramidal to linear. Step 1 We need to count the valence electrons of the xenon tetrafluoride molecule with the help of a periodic table.
Drawing the Lewis Structure for XeO 2 F 2. So you have a total of 8146 28 valence electrons. Draw lewis structure of HCHO.
Now we must figure out how many valence electrons total we would need to make every atom happy octet rule All four atoms want eight electrons so we need a total of 4x832 electrons. Draw lewis structure of XeF4. Draw the Lewis dot structure for eqXeF_2 eq.
The Lewis structure for XeO 2 F 2 requires you to place more than 8 valence electrons on Xe. Ib chemistry 4-5 metallic bonding. There are Total of 22 valence electron in the Lewis structure for XeF 2.
Lewis dot structure is a representation of a covalent molecule in which the distribution of electrons on atoms is shown as dots. This tells us that there are five electron regions Steric Number 5 about the central carbon atom. Use lewis structure guidelines to draw the lewis structure of phosphate ion.
Abdul Changed status to publish 1 hour ago. For the above molecule VSEPR notation will be AX 2 E 3. After calculating number of valence electron distribute them around the center atom.
Draw the Lewis structure 3 marks B.

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Introduction To Molecular Geometry Organic Chemistry

Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons
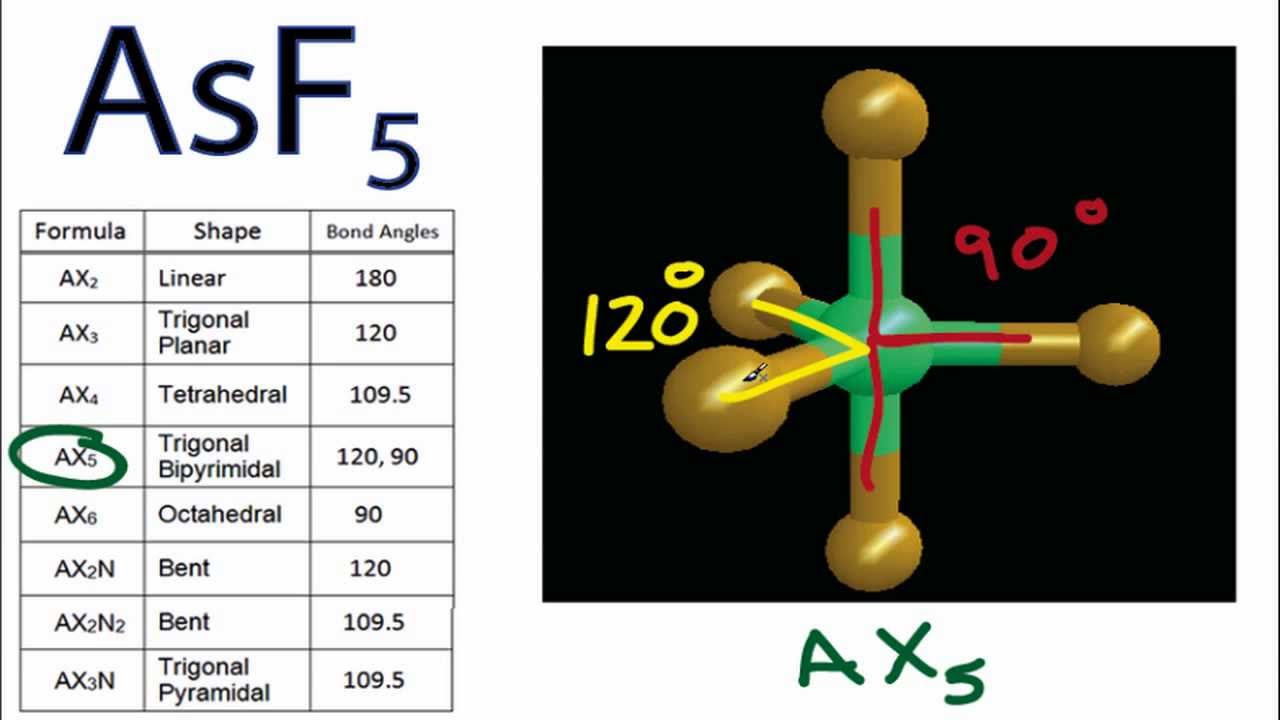
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Xef2 Lewis Structure Xenon Difluoride In 2021 Lewis Molecules Math Equations

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Introduction To Molecular Geometry Molecular Geometry Molecular Geometry
