Expanded Octet Lewis Structure For Pcl5
The first step is to draw phosphorous PP in the center. Atoms in pe.

The Expanded Octet Introduction To Chemistry
Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table.
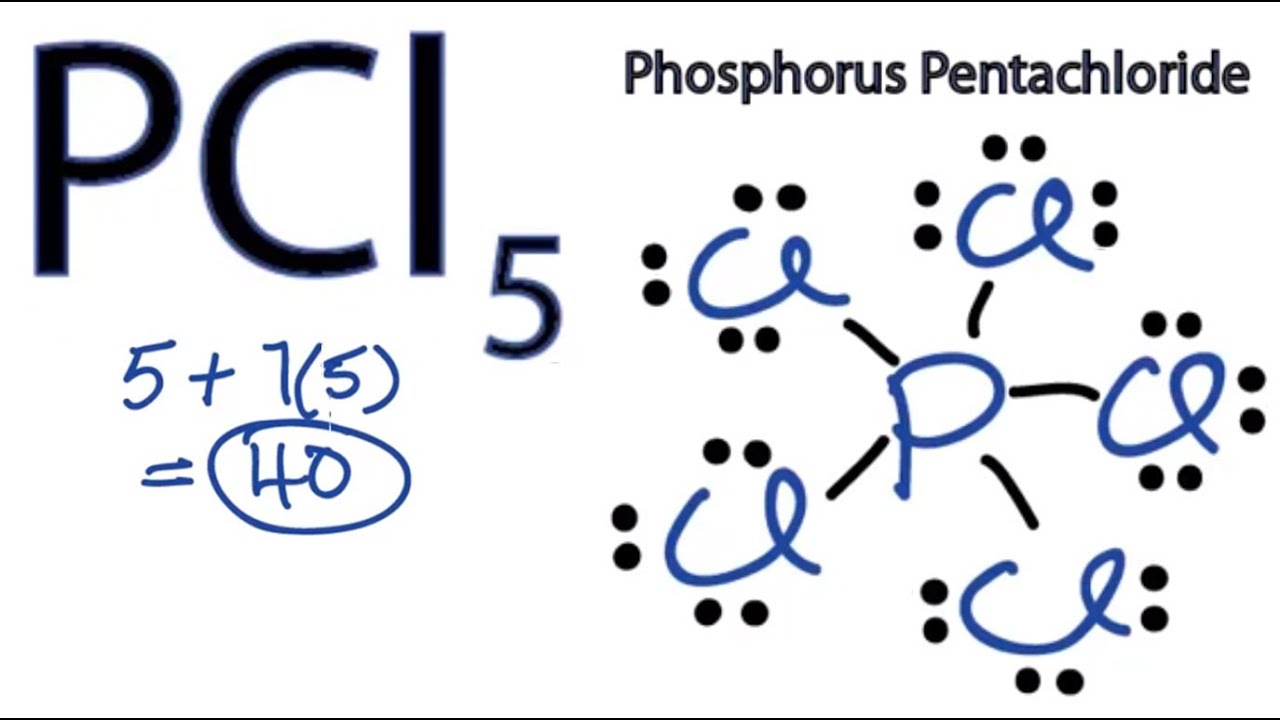
Expanded octet lewis structure for pcl5. Expanded Octet in Lewis Structures Expanded octet refers to the Lewis structures where the central atom ends up with more than an octet such as in PCl 5 or XeF 4. The overall geometry of the molecule is depicted trigonal bipyramidal and bond angles and lengths are highlighted. How to determine the Lewis structure for PCL5 -.
Always start by drawing out the lewis structure for these two. Phosphorus has five valence electrons and each chlorine has seven valence electrons so the Lewis electron structure of PCl 5 is. 7911024 Solution p373 the lewis structure for pcl5 is shown.
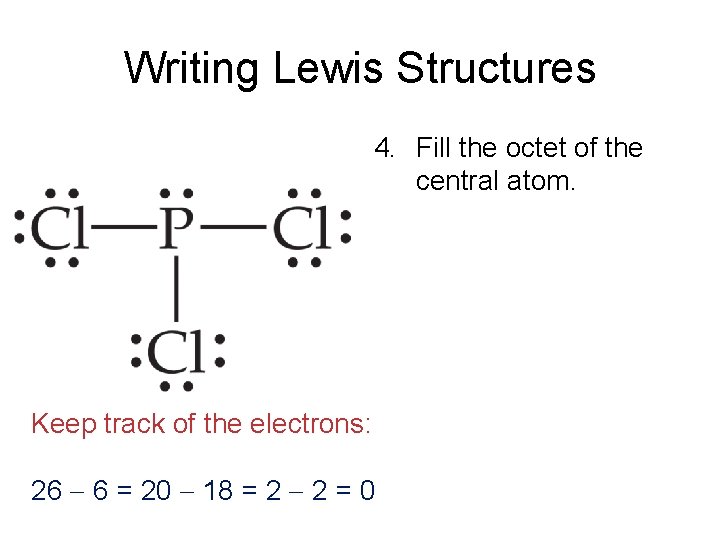
Alternatively a dot method can be used to draw the lewis structure. Lewis Structure Expanded Octet Step 4A. Lewis dot structure of PCl5.
This means that it can hold more than 8 valence electrons. Explain the structure of pcl3 and pcl5 - Chemistry. As a result the second period elements more specifically the nonmetals C N O F obey the octet rule without exceptions.
But the molecule PCl5 overall is a nonpolar chemical compound due to its Cl atoms forming a Trigonal Bipyramidal structure. MathrmPCl_5 Announcing Numerades 26M Series A led by IDG Capital. Back to Molecular Geometries Polarity Tutorial.
Draw the expanded octet Lewis structure for each molecule. Read how Numerade will revolutionize STEM Learning. Expanded octet refers to the Lewis structures where the central atom ends up with more than an octet such as in PCl5 or XeF4 In drawing the Lewis structurenbsp.
Expanded octet PCl5 Janet Coonce takes. Answer the questions in the table below about the shape of t. Expanded octet PCl5 Duration.
Expanded octet PCl5 beach inspired bedrooms pcl5 lewis structure fusion stock images pcl5 lewis structure Pcl5 Lewis Structure. Expanded octet PCl5 Duration. For example a lone pair.
Helpful tool for pcl. This property underpins many of its. Lewis Structures Expanded Octet Pcl5 Janet Gray Coonce Which lewis structure or structures are most appropriate according to the formal charges.
In drawing the Lewis structure for PCl 5 there is a total of 40 valence electrons to put in 5 5x7 40. Step method to draw lewis structure of phosphorous penta chloride. SCl2 PH3 CO2 N2 ClF5 PCl5 XeF2.
Scl2 Ph3 Co2 N2 Clf5 Pcl5 Xef2 The structure of pcl5 depends on its environment. The 5 chlorine Cl. Alternatively a dot method can be used to draw the lewis structure.
Drawing the Lewis Structure for PCl. Apply VSEPR notation A X E ANumber of. There are 2 axial chlorines at the top and bottom of the Pcl5 Lewis Structure.
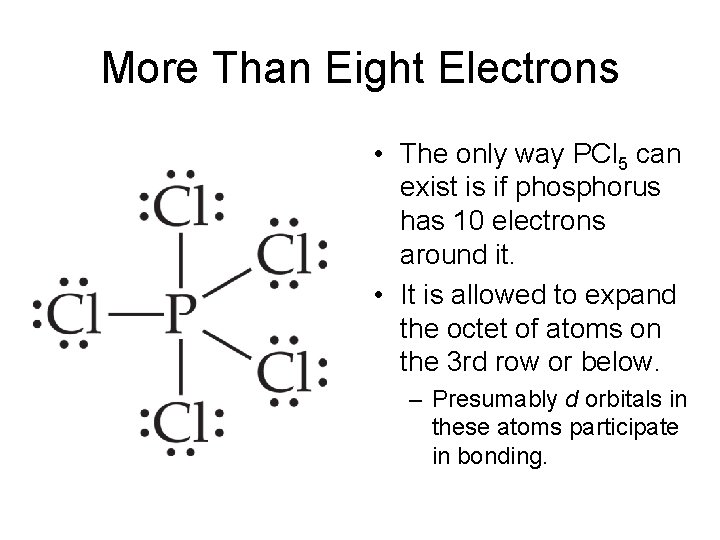
One can easily see that if the central atom P is to be joined to five. Molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule. Clf is lewis structure do a question showing Draw the Pcl5 Lewis Structure.
Explain the geometery shape of molecule Pcl5 and Pcl3 step. Lewis Symbols and Structures Introductory Chemistry. Lewis dot structure of PCl5.
This is why chemists laugh at students when they draw carbon with more than 4 bonds. Expanded octet PCl5 - Duration. What is the Lewis Structure of PCl5.
Lewis dot structure of PCl5. Place one lone pair on the center P atom to reach Draw the Lewis Dot structure for RbIO2 Brainly Introduction to Chemical Bonding Chemistry LibreTexts Exceptions to the Octet Rule Chemistry LibreTexts. Use VSEPR table to find the shape.
Phosphorus pentachlorideIn the PCl 5. Dipole moment d lewis structure for bonding molecular Side view of PCl5. Exceptions to the Octet Rule.
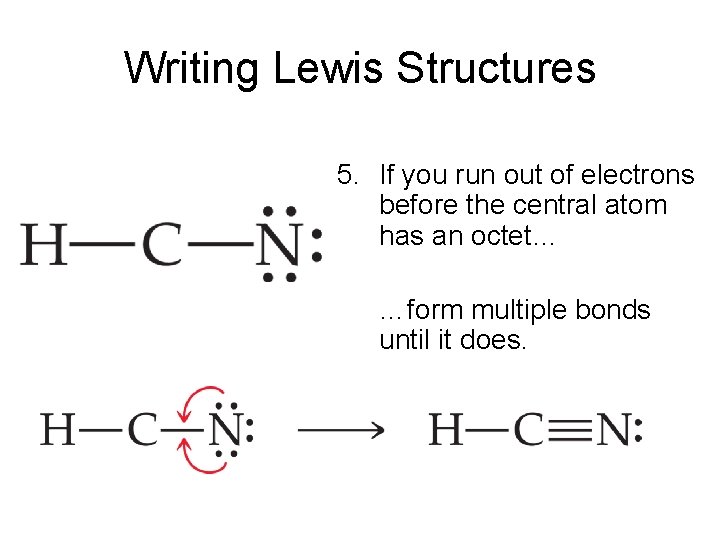
Nov 7 2017 - Atoms in periods 12 cant have an expanded octet. Five pairs will be used in the chemical bonds between the P and Cl. Minnie Huff Moreover by sharing a bonding pair with oxygen each hydrogen atom now has a full valence shell of two electrons.
5 There are a total of 40 valence electrons in the PCl5 Lewis structure. Up next Lewis structures. 426306 Pocl has a single bond to cl and a double.
Expanded octet PCl5 Connect to WiFi to prevent cellular charges for video streaming Now lets draw the Lewis dot structure for PCl5 phosphorous pentachloride. Phosphorus has five valence electrons and each chlorine has seven valence electrons so the Lewis electron structure of PCl 5 is. Step method to draw lewis structure.
Lewis Structures - Chemistry LibreTexts. Pcl 5 Lewis Structure.
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Chemistry Chemical Bonding 31 Of 35 Lewis Structures Exception To The Expanded Octet Rule Helpful Video When Get Octet Rule Secondary Science Chemistry
What Is An Expanded Octet Quora

The Expanded Octet Introduction To Chemistry
How To Determine The Lewis Structure For Pcl5 Quora

How To Draw The Lewis Structure Of Pcl5 Phosphorus Pentachloride Youtube

Expanded Octet In Lewis Structures

Covalent Bonding Lewis Structures Ppt Video Online Download
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

How To Determine The Lewis Structure For Pcl5 Quora
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Brief Answer And Explanation Of This Question Please Why Does Pcl 5 Exist But Ncl 5 Does Not Use The Lewis Structure Of Each To Explain Your Answer What Is The Molecular Shape Of

Exceptions To The Octet Rule Lewis Dot Diagrams Youtube

Lewis Structures Expanded Octet Pcl5 Janet Coonce Takes Time To Draw This Diagram Step By Step A Gre High School Science Secondary Science Teaching Science
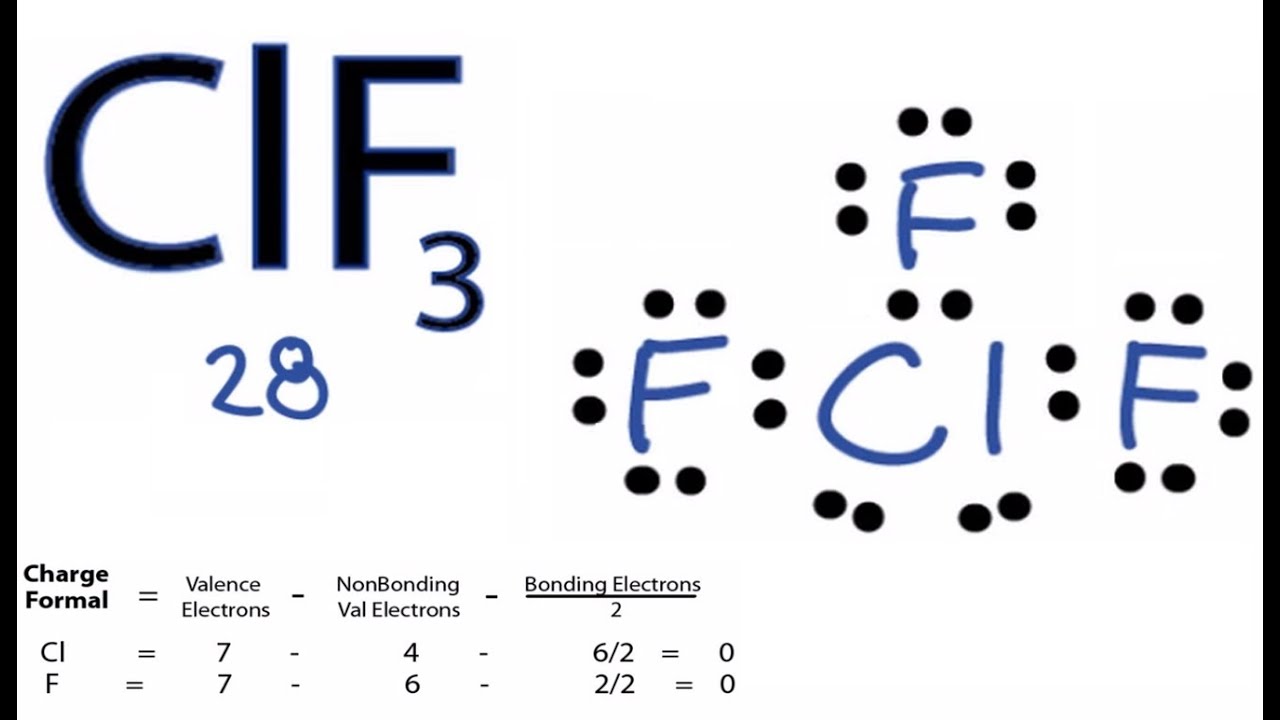
Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube
Chem 101 Octet Rule Violations

Exceptions To The Octet Rule Boundless Chemistry
