H2o Lewis Structure And Vsepr Model
Write or type the electron and molecular geometries. Draw the Lewis structure for H2O.

H2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
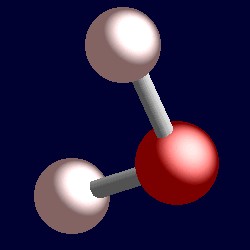
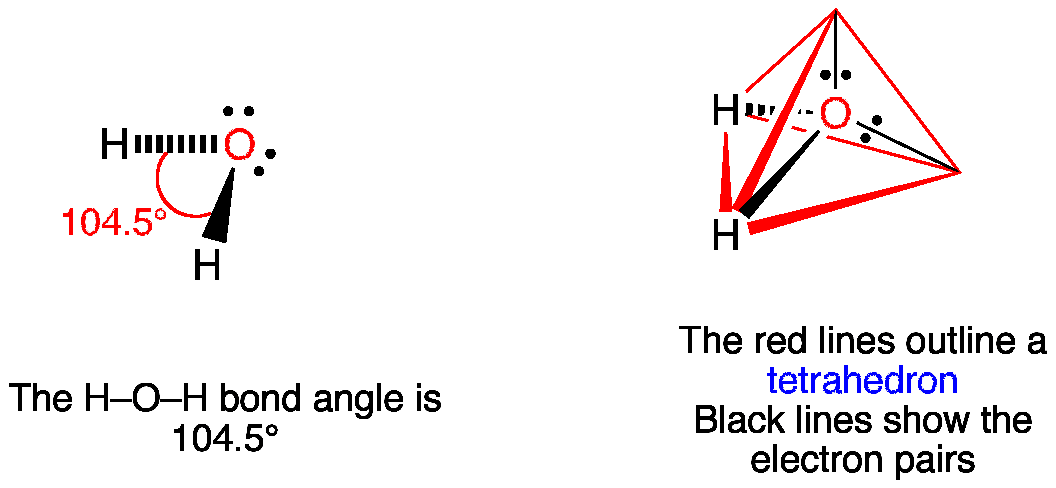
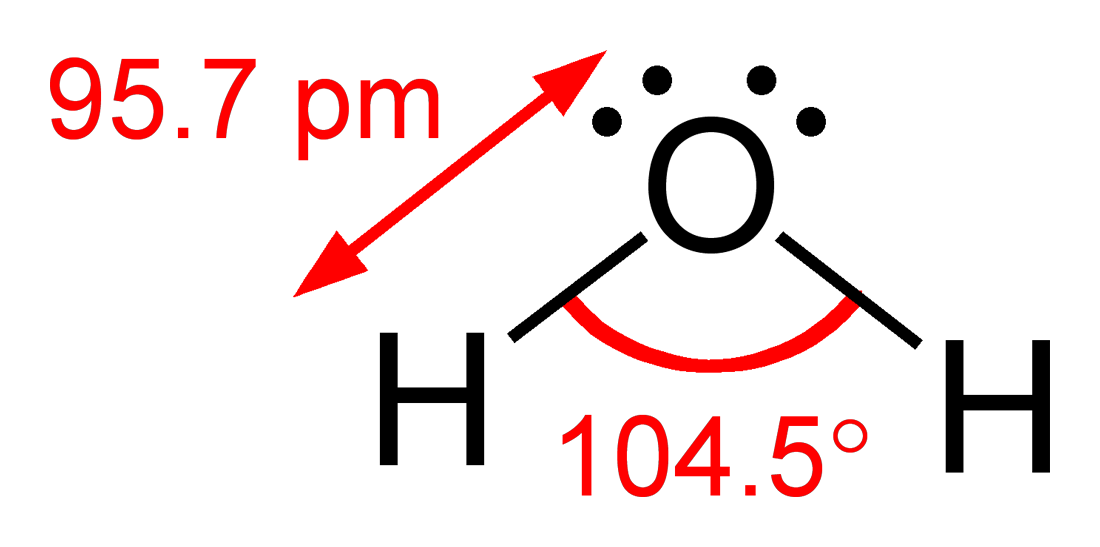
The bond angle is 1045 0 which is less than ideal for tetrahedral geometry 1095 0 due to presence of two lone lone pairs.
H2o lewis structure and vsepr model. How many lone pairs of electrons need to be added to complete this Lewis structure. Use lewis structure guidelines to draw the lewis structure of PCl 5. Valence Shell Electron Pair Repulsion VSEPR theory along with Lewis structures can be used to predict molecular geometry.
First draw the Lewis structure of water. Given a Lewis structure distinguish between bonded pairs and non-bonded pairs of electrons 3. These are arranged in a tetrahedral shape.
Draw Lewis structures of polyatomic molecules and ions 2. On the basis of VSEPR theory what is the structure of H2O and ammonia NH3 molecules B-Sc. Thus the electron-pair geometry is tetrahedral with three of the corners occupied by the bonding pairs of electrons.
According to the VSEPR model a molecule with the general formula AB2 with two lone pairs on the central atom will have a ___ molecular geometry. Linear square planar tetrahedral bent octahedral. For showing the sharing of electrons show a single bond on both sides.
Since the steric number is 4 and there are two lone pairs water has bent geometry. VM-CA2 where V- Noof Valence electrons of the central atom. The VSEPR theory assumes that each atom in a molecule willachieve a geometry that minimizes the.
To find the structure of a molecule we have a formula. 022 - Lewis Diagrams and VSEPR ModelsIn this video Paul Andersen explains how you can use Lewis Diagrams and VSEPR Models to make predictions about molecules. The central Sn atom is surrounded by one nonbonding electron pair and three single bonds.
There is no direct relationship between the formula of acompound and the shape of its molecules. What is the predicted molecular geometry of the H2O molecule according to the VSEPR model. As a result there are two lone pairs in this molecule and two bonding pairs of electrons.
It is based on the assumption that pairs of electrons occupy space and the lowest-energy structure. M- Noof monovalent atom attached to central atom. Lewis structures of H 2 O and SO 2.
The electronic structure of molecules can be illustrated by Lewis structures which can be used to and properties such as geometry bond orders bond lengths relative bond energies and dipoles. Question 7 1 point Listen Using VSEPR model how is the electron arrangement about the central atom electron-pair geometry for H2O. 1 56 points Use the VSEPR model to supply the following information.
The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. Lewis electron structures give no information about molecular geometry the arrangement of bonded atoms in a molecule or polyatomic ion which is crucial to understanding the chemistry of a molecule. H2O has two bond pairs and two lone pairs total four electron density groups.
Draw resonance structures for cases where more than one valid Lewis structure is possible 4. Use the Valence Shell Electron Pair Repulsion VSEPR model to predict the geometries of molecules and. The resulting molecular shape is bent with an H-O-H angle of 1045.
Using the VSEPR model predict the molecular geometries of. Draw the Lewis structure. According to this model valence electrons in the Lewis structure form groups which may consist of a single bond a double bond a triple bond a lone pair of electrons or even a single unpaired electron which in the VSEPR model.
Determine the number of electron groups the electron geometry and the molecular shape. SOLUTION a The Lewis structure for the SnCl 3-. The shape of the molecule is bent although the geometry is tetrahedral.
Where two or more resonance structures can represent a molecule the VSEPR model is applicable to any such structure. Draw the Lewis structure for H2O. This is the Lewis structure of the H 2 O molecule that has two single bonds between Oxygen and Hydrogen.
How many lone pairs of electrons are there in the central atom. Both Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure. Circle the approximate bond angles hybridization of the central atom and the polarity of the molecule PCL Electron domain geometry Molecular geometry Approximate bond angles 1095 90.
The shapes of thesemolecules can be predicted from their Lewis structures howeverwith a model developed about 30 years ago known as the valence-shellelectron-pair repulsion VSEPR theory. The following table shows VSEPR structures based on a compounds steric number and the number of lone pairs. Since the O atom has two bonds and two lone pairs its steric number is 4.
Water has 4 regions of electron density around the central oxygen atom 2 bonds and 2 lone pairs.
Question 2 1 Point Draw The Lewis Structure For Chegg Com
What Is The Shape And Geometry Of H2o And Xef4 Quora
What Is The Shape And Geometry Of H2o And Xef4 Quora
Of2 Lewis Structure Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

H2o Molecular Geometry Shape And Bond Angle Precise Angle Is 104 45 Youtube
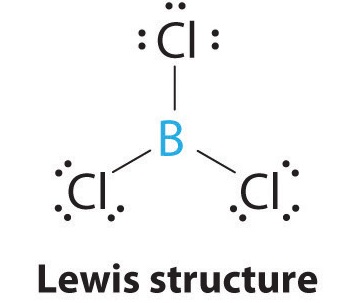
10 3 Vsepr Geometry Chemistry Libretexts
The Vsepr Theory To Predict The Electronic And Molecular Geometry

6 3 Molecular Shape Introductory Chemistry

H2o Molecular Geometry Lewis Structure Shape And Bond Angles

Vsepr For 4 Electron Clouds Video Khan Academy

Molecular Geometry Boundless Chemistry
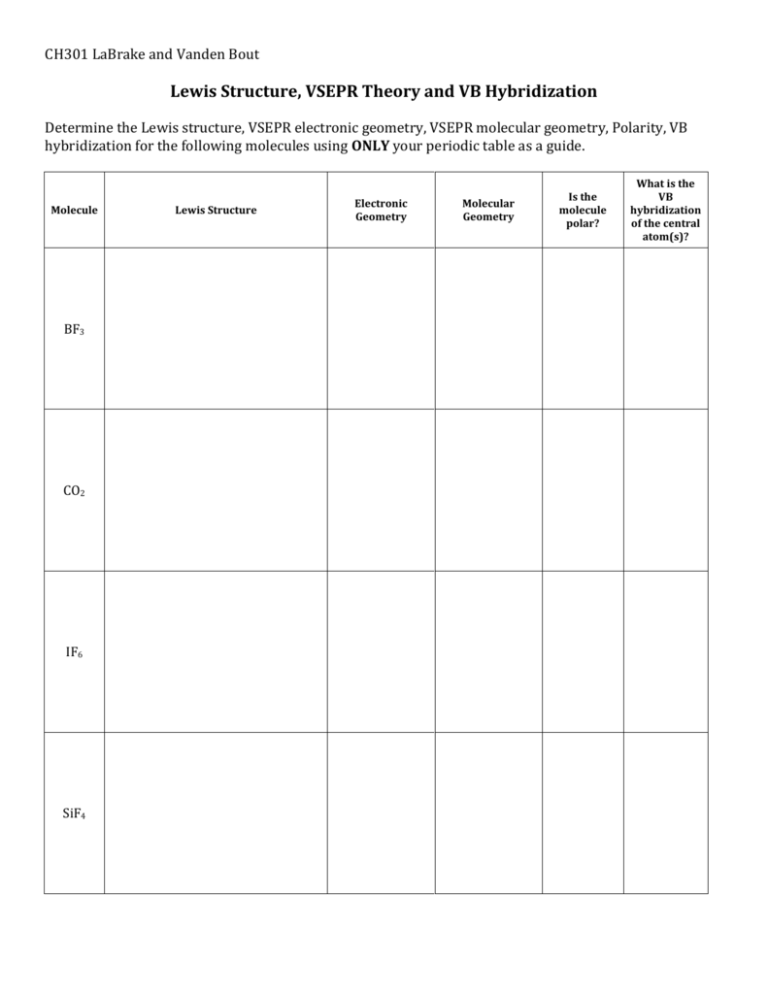
Lewis Structure Vsepr Theory And Vb Hybridization Wkst

Valence Shell Electron Pair Repulsion Theory Vsepr

Water Molecular Geometry And Bond Angles Youtube
H2o Molecular Geometry Lewis Structure Shape And Bond Angles
Vsepr Theory And Molecular Geometry Ch4 Nh3 H2o Youtube