Is Sulfuric Acid A Lewis Acid
Explore the latest full-text research PDFs. Because HF is a weak acid fluoride salts behave as bases in aqueous solution.
Sulfuric acid is a dibasic strong acid.
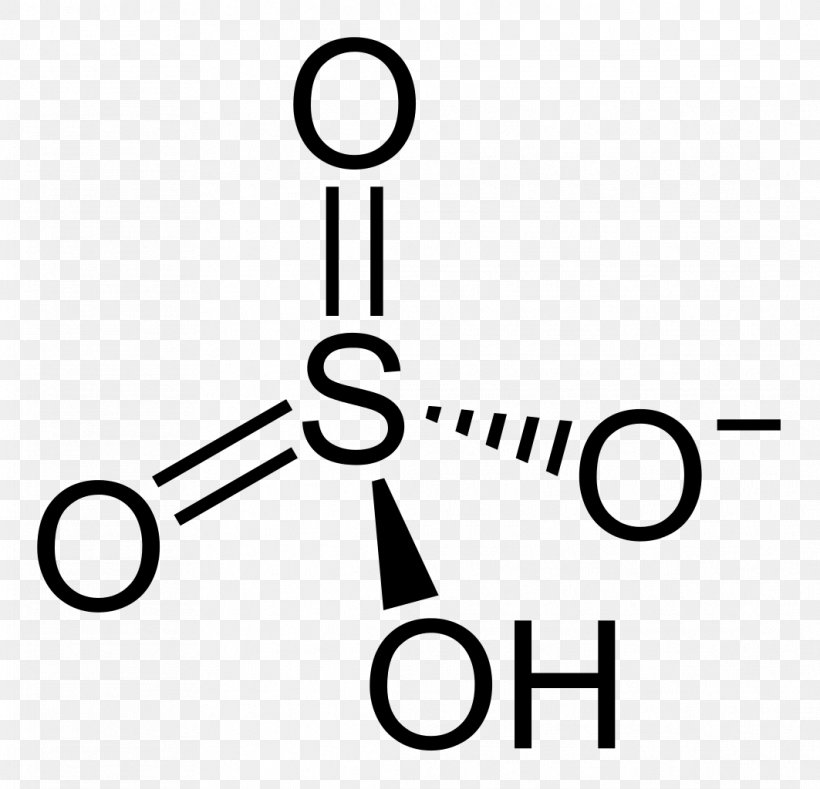
Is sulfuric acid a lewis acid. Total valence electrons concept is used to draw the lewis structure of H 2 SO 4. Sulfuric acid can be described as all of the following EXCEPT. The bisulfite ion is amphiprotic and can act as an electron donor or acceptor.
A strong electrolyte a Lewis acid a diprotic acid an amphoteric compound The answer was amphoteric compound which I got. However the conjugate base of sulfuric acid bisulfate anion is itself a reasonably strong Bronsted acid. 1 Gas drying 2 Catalytic conversion of sulfur dioxide to sulfur trioxide 3 Absorption of sulfur trioxide and 4 Acid cooling.
In the special case of aqueous solutions proton donors form the hydronium ion H3O and are known as Arrhenius acids. H SO 4 H 2O SO2 4 H 3O. Almost without exception contact plants operate under essentially atmospheric.
This clearly cant be right because I have never seen a sulfuric acid Lewis structure with two lone pairs. Sulfuric acid H 2 SO 4. An acid is a molecule or ion capable of either donating a proton known as a BrønstedLowry acid or capable of forming a covalent bond with an electron pair known as a Lewis acid.
It is a strong and highly corrosive mineral acid that is miscible with water so it can be prepared in the form of solutions. There are four main process steps in the production of sulfuric acid from sulfur dioxide-containing gases by the contact process. Sulfuric acid is a strong dibasic acid.
The salts and esters of sulfuric acid are known as SULFATES and SULFURIC ACID ESTERS. I struggled with this one though because I did not see how it could act as a Lewis acid either. Therefore we can assume there should be two -OH bonds in sulfuric acid molecule.
Its molecular formula is H 2 SO 4 and it is by far the most widely produced and used mineral acid in the world. The first category of acids are the proton donors or BrønstedLowry acids. Sulfuric acid H2SO4 consists of 2 oxo- groups and 2 hydroxy- group is a high corrosive acid to metals and tissuesIt poses healthy risk from inhalationIt.
Give an example of a Lewis acid-base reaction that does not involve protons. Nucleophile attacks electrophile but that gives the central sulfur two lone pairs of electrons. The gas-drying stage is not applicable to a plant of the wet-catalysis type.
The sulfuric acid also called hydrogen sulfate and oil of vitriol is an acid oxyacid of sulfur formed by the reaction of sulfur trioxide SO 3 and water. PS I once asked a Scandinavian who was an excellent. As a Lewis base F accepts a proton from water which is transformed into a hydroxide ion.
Write equations illustrating the behavior of a given non-aqueous acid-base system. Inorganic and organic derivatives of sulfuric acid H2SO4. The Brønsted-Lowry proton donor-acceptor concept has been one of the most successful theories of Chemistry.
It means it can release two hydrogen atoms to show acidic characteristics. This reaction goes to completion in water. But as with any such theory it is fair to ask if this is not just a special case of a more general theory that could encompass.
Overall H 2SO4aq 2H 2Ol 2H 3O SO2 4. As in any chemical reaction both MASS and CHARGE are conserved. I went to Wikipedia and it says the sulfur trioxide molecule has 2 dative bonds and one double bond.
This seems to make a little bit more sense.

Safety Data Sheet Nitrosyl Sulfuric Acid

File Sulfuric Acid Lewis Png Wikimedia Commons

Dissolution Mechanism Of Cellulose In 72 Wt Sulfuric Acid Based On Download Scientific Diagram

Sulfuric Acid Podcast Chemistry World

Strong Acids And Strong Bases Acids And Bases That Are Strong Electrolytes Completely Ionized Teaching Chemistry Chemistry Education Organic Chemistry Study
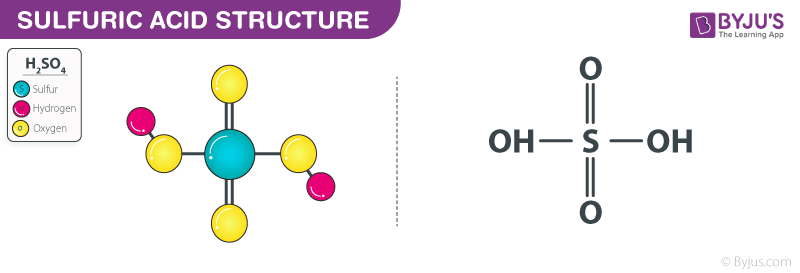
Sulfuric Acid H2so4 Structure Molecular Mass Properties Uses
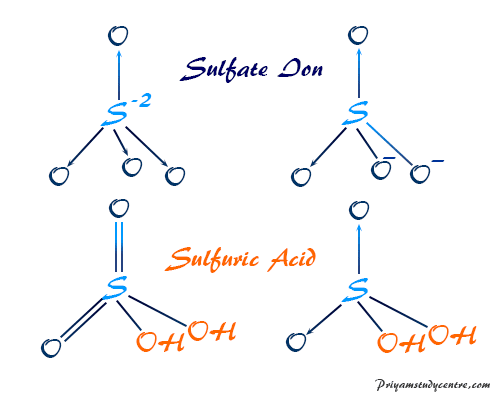
Sulfuric Acid H2so4 Structure Production Properties Uses
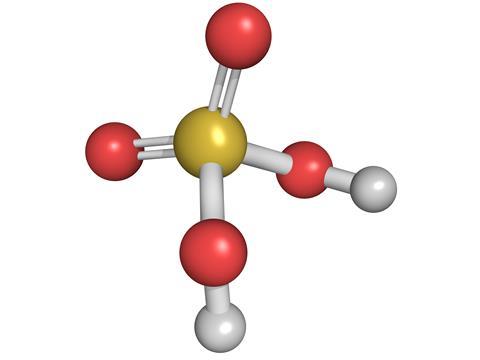
Sulfuric Acid Podcast Chemistry World

Ppt Sulfuric Acid H 2 So 4 Powerpoint Presentation Free Download Id 2067709

Electrophilic Aromatic Substitution Nitration Of Benzene Organic Chemistry Chemical Structure Chemistry

Sulfuric Acid Mineral Acid Lewis Structure Sulfate Png 1063x1024px Watercolor Cartoon Flower Frame Heart Download Free

Dissolution Mechanism Of Cellulose In 72 Wt Sulfuric Acid Based On Download Scientific Diagram
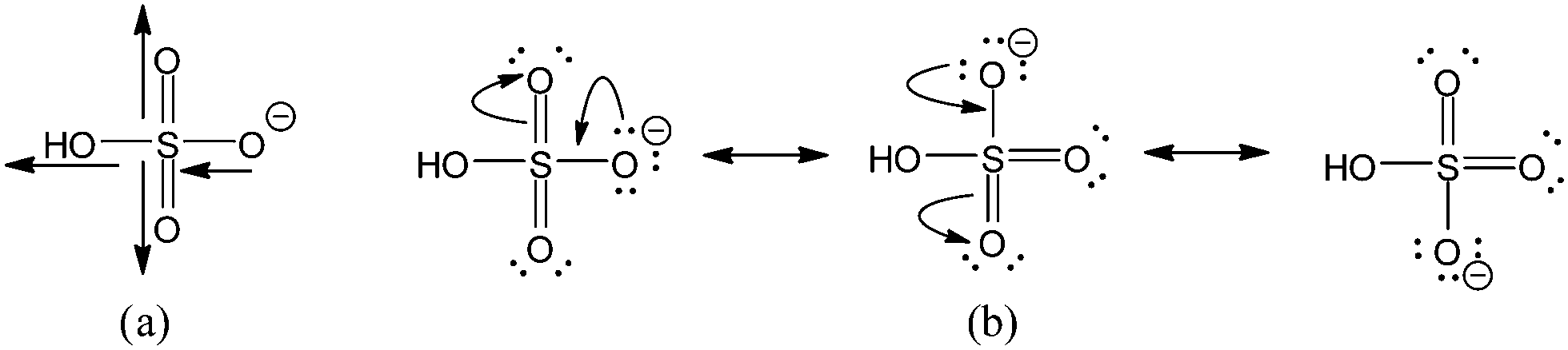
Why Is Sulfuric Acid A Much Stronger Acid Than Ethanol Determination Of The Contributions By Inductive Field Effects And Electron Delocalization Effe Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cp04110k
Sulfuric Acid H2o4s Chemspider
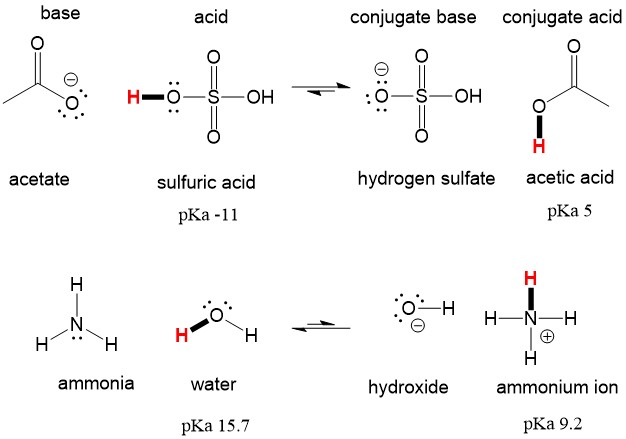
Reviewing Acid Base Definitions Organic Chemistry Help



