Lewis Diagram Of Pcl3
70 More Lewis Dot Structures. I quickly take you through how to draw the Lewis Structure of PCl3 phosphorous trichloride.

What Is The Molecular Geometry Of Pcl3 Study Com
One is phosphorous P and the second is chlorine Cl.

Lewis diagram of pcl3. PCl3 lewis structure In this lewis structure of PCl3 center phosphorus atom has made three single bonds with three chlorine atoms. Draw the Lewis structure of SO24. The A represents the central atom the phosphorus each X represents a chlorine atom and the E represents the lone pair.
It has a pyramidal shape as shown in which phosphorus is sp 3 hybridised. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction ca. To draw the PCL3 lewis structure follow the below instructions.
Also there is a lone pair on phosphorus atom. Include all the lone pairs. Also there are no charges on atoms in PCl3 lewis structure.
How to Draw the Lewis Structure of PCl3 phosphorus trichloride. First of all find out the total number of valence electrons in the PCL3 using the periodic table. Which in turn enjoy.
Valence electrons of Phosphorus Valence electrons of Chlorine. PCl 3 is important indirectly as a precursor to PCl 5 POCl 3 and PSCl 3. In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride.
In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. Draw the Lewis structure of PCl3. Now lets move on to the lewis structure of PCl3.
To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms. Lewis symbols can also be used to illustrate the formation of cations from atoms as shown here for sodium and calcium. Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms.
Figure 2 demonstrates the use of Lewis. Write Lewis structures for the following. I also go over hybridization shape and bond angle.
5 73. Likewise they can be used to show the formation of anions from atoms as shown below for chlorine and sulfur. Science Chemistry Lewis structure.
What is the Lewis dot structure for PCL3. The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. You can watch me draw the Lewis Dot Diagram for PCl3 here.
These chlorines want to satisfy their oxide requirement and that is why the geometry for PCL3 is called Trigonal Pyramidal. Draw the Lewis structure of SO24. Here Phosphorous 5 valence electrons Chlorine 7 valence electrons 3 Cl 73 21 So total valence electrons 26.
1 Structure of PCl 3. Include all the lone pairs. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Draw the lewis structure of PCl3. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3.
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. 2 Structure of PCl 5. What is the structure of PCl3.
The lewis structure of PCl3 can be explained as follows. Draw the Lewis structure for PCl3 phosphorus trichloride which is used in the preparation of pesticides and flame retardants. It has a trigonal bipyramidal structure in gaseous and liquid phases.
In the Lewis structure of PCL3 there are two chemical compounds. The three equatorial PCl bonds are equivalent while the two axial bonds are longer than equatorial bonds. Draw the Lewis structure of PCl3.
Answer all questions related to the drawing Underneath draw the lewis structure What is the geometric shape For the central atom what is the formal charge What is the hybrid orbital designation for the central atom How many sigma bonds in the formula How many pi bonds in. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off. In this tutorial we will learn how to draw the lewis structure of PCl.
Total number of valence electrons of PCl3. When we examine the Lewis structure of PCL3 we can see that each chlorine atoms have 3 lone pairs and all of them must have 8 electrons around it. What shape is bf3.
What Is The Bond Angle Of Pcl3 Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Is The Electron Dot Structure Of Pcl3 Determined Quora

Lewis Dot Structure Easy Hard Science

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Pcl3 Lewis Structure Phosphorus Trichloride Youtube

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

What Is The Molecular Shape Of Pcl3 Quora
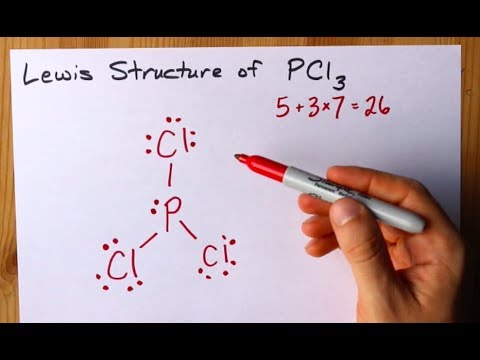
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Is Pcl3 Polar Or Nonpolar All About Pcl3 Polarity Structure
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
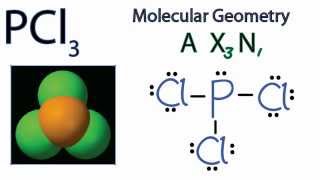
Pcl3 Molecular Geometry Shape And Bond Angles Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
How Many Lone Pair Electrons Are There In Pcl3 Quora
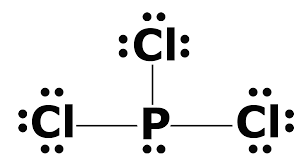
Solutions 17 Chemistry Libretexts

What Is The Lewis Dot Structure For Pcl3 Study Com
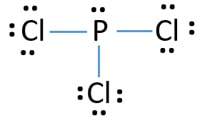
Pcl3 Phosphorus Trichloride Lewis Structure
