Lewis Dot Structure For Sif6 2-
Be sure to put brackets and a 2- around the SiF 6 2-Lewis structure to show that it is an ion. Draw The ELECTRON DOT Structure.

Lewis Dot Of The Silicon Hexafluoride Ion Sif6 2
It will hold more than 8 electrons.

Lewis dot structure for sif6 2-. Silicon Si is the least electronegative and goes at the center of the Lewis structure. Orthosilicate O4Si-4 CID 104812 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Draw The ISOMERS F.
CO 3 2 4. NOCl CF 2 Cl 2 HCN. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom.
Advanced Lewis Structures And Molecular Geometry For Each Of The Following Moleculesions A. Find the total valence electrons for the molecule. The Lewis electron dot structures of a few molecules are illustrated in this subsection.
See the answer See the answer See the answer done loading. 2 x S 6 x 2 12. Step 2Arrange the atoms.
Experts are tested by Chegg as specialists in their subject area. CH 2 Br 2 d. Steps for Writing Lewis Structures.
I quickly take you through how to draw the Lewis Structure of SF6 2- Silicon HexaFluoriode Ion. H always goes outside. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and.
Used to make other chemicals. Determine ELECTRON PAIR GEOMETRY And HYBRID ORBITALS Used On Central Atoms Using VSEPR Theory C. H 2 S c.
May be toxic by ingestion. NO 3 d. H 2 S NCl 3 OH -.
Lewis Structure of CO2. Draw the Lewis structure for the SnF 6 2- ion. Draw resonance structures of some molecules.
Lewis Structure Examples. Appears as a crystalline solid or the solid dissolved in a liquid. The Lewis Dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons.
Lets produce a Lewis Dot structure for carbon disulfide CS 2Step 1. Thus SF6 has 48 valence electrons that will help us draw the Lewis Dot Structure of SF6. Put the least electronegative atom in the center.
A step-by-step explanation of how to draw the SiF62- Lewis Dot StructureFor the SiF6 2- Lewis structure calculate the total number of valence electrons for. This structure helps us know the bond formations in the molecule and the arrangement of electrons in it. Fluorine Group VII which means it has a total of seven 7 valence electrons around the atom.
For the SF6 Lewis structure you should take formal charges into account to find the best Lewis structure. Total 4 12 16 e8 electron pairs. Quiz your students on SiF6 2- Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching.
Assess the stability of a structure by considering formal charges of atoms. There are a total of 48 valence electrons in SiF 6 2-. NH 4 c.
Contact may irritate skin eyes and mucous membranes. Draw the Electron dot structure. Hexafluorosilicate 2- is a silicon coordination entity.
Who are the experts. Put two electrons between atoms to form a chemical bond. Draw the Lewis dot structure of a given molecule or ion.
We review their content and use your feedback to keep. Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. I also go over hybridization shape and bond angle.
Assign formal charge to an atom in a dot structure. Go with Carbon in the centerStep 3Fill in the electron pairsStep 4Check for the octet rule. Silicon having valence electrons in the 3rd energy level will also have access to the 4d sublevel thus allowing for more than 8 electrons.
Draw the Lewis Dot Structure. Draw the Lewis dot structures for each of the following molecules. PO 4 3 b.
Si does not follow the octet rule. This problem has been solved. For the following molecules or ions where the central atom is underlined.
Since Fluorine is in Period 2 it can fit a maximum of eight 8 electrons second energy level. Draw the Lewis Dot Structure for the Florine atom. SiF 62- is d 2 sp 3 hybridized and contains no lone pair and 6 bonding pairs of valence electrons around the Silicon.
Since it is bonded to only one carbon atom it must form a double bond. Draw the Lewis structure for the SnF62- ion. Determine The GEOMETRY Of The Moleculeion D.
70 More Lewis Dot Structures. Draw the Lewis dot structure for each of the following polyatomic ions. Oxygen contains 6 valence electrons which form 2 lone pairs.
Draw The RESONANCE Forms E. The central atom of this molecule is carbon. There are a total of 48 valence electrons in the Lewis structure for SF6.
Silicon Si is below Period Two on the periodic table and can hold more than 8 valence electrons. XeO 2 F 2.
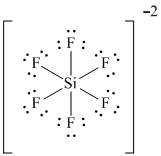
Solved Write A Lewis Formula For The Anion Sif6 2 That Would Be Chegg Com

Draw The Electron Dot Structure For Sbr 2 Study Com

Ascl3 Lewis Structure How To Draw The Lewis Dot Structure For Ascl3 Youtube

Construct Electron Dot Diagrams For A Th Clutch Prep

Sf6 Molecular Geometry Lewis Structure Shape And Polarity
Question 5 2 F F Si F F What Is The Chegg Com

How To Draw Lewis Structure For Sf6 Drawing Easy

Start With The Lewis Dot Structure And Then Draw The Chegg Com

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Lewis Structures 100 Lewis Structures
Kawbikfdwq Sif6 2 Lewis Structure Example

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube
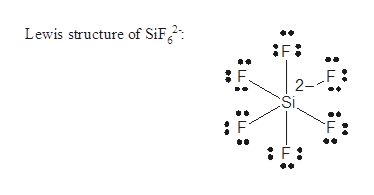
Answered Sif62 Nsf F2co Fno2 Deduce The Bartleby

Draw The Lewis Structure For Sif62 Study Com
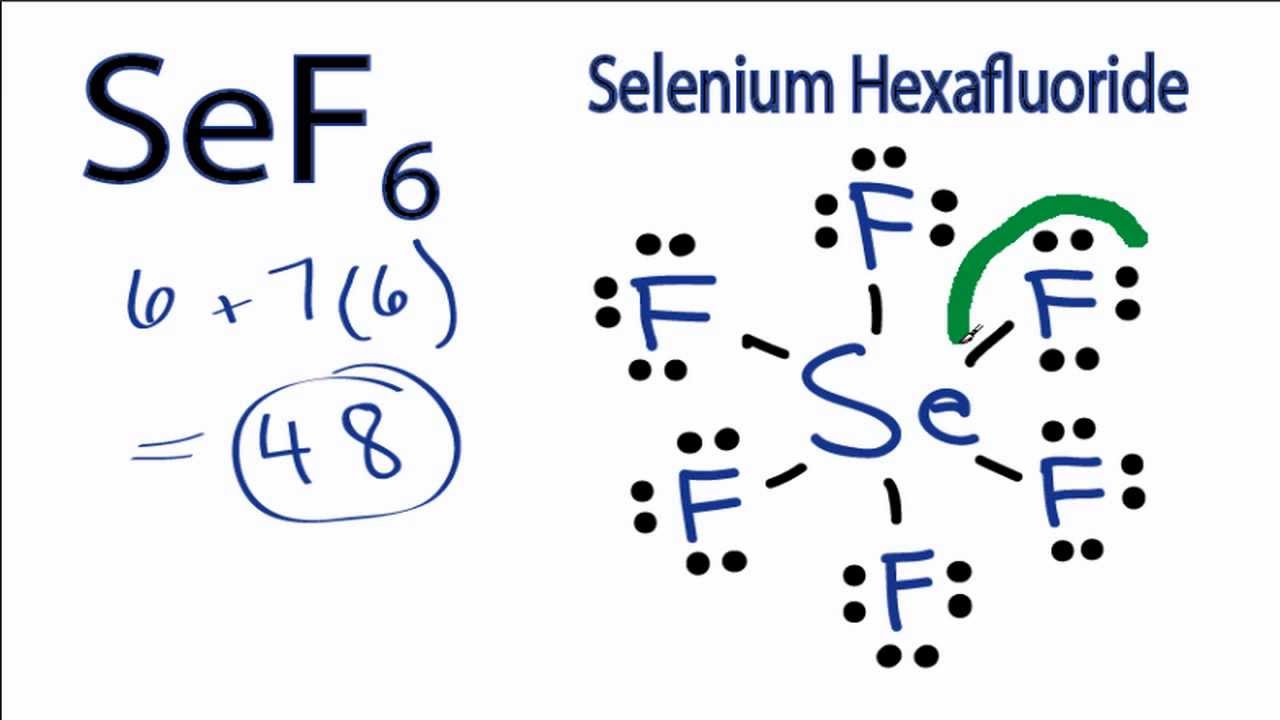
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube
How To Draw Lewis Structure For Sf6 Drawing Easy

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube
Kawbikfdwq Sif6 2 Lewis Structure Example

