Lewis Structure For So2 Resonance
How do you know if a structure has resonance. It discusses the molecular geometry.
File Sulfur Dioxide Ve Resonance 2d Png Wikipedia
Resonance structures are a set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges.

Lewis structure for so2 resonance. Resonance structure of so2 Complete the other resonance structure by dragging bonds and lone electron pairs to their appropriate positions. It discusses the molecular geometry bond angle. A step-by-step explanation of how to draw the HSO4- Lewis Dot Structure Bisulfate ion or Hydrogen sulfate ionWhen we have an H or H2 in front of a polya.
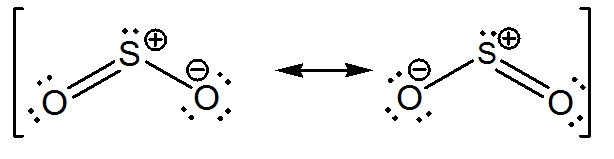
And then we have a lone pair of. All resonance structures must follow the rules of writing Lewis Structures. SO2 Resonance Structures The sulfur dioxide SO 2 Lewis structure is shown below.
We have three different structures differing ONLY in the locations of the electrons. Write Lewis structure of the following compounds and show formal charge on each atom. For the CO2 resonance s.
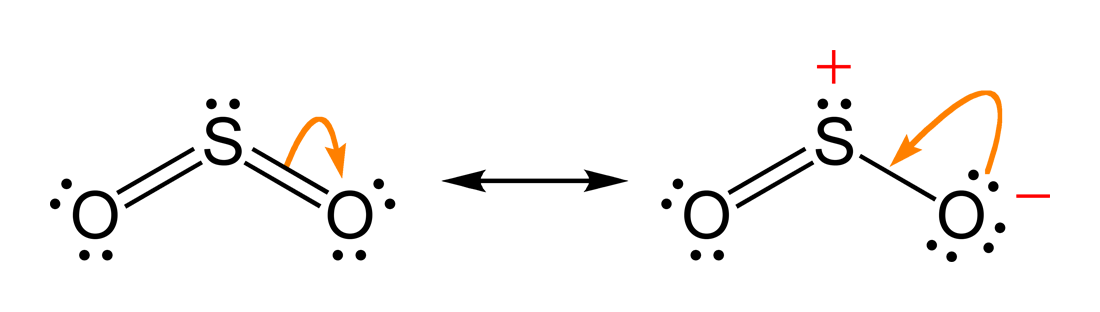
A singled bonded oxygen atom a double bonded oxygen atom and a pair of dots shown on top. The reason is that the octet rule is observed this way no hybridization of d-orbitals for main group chemistry necessary or possible as well as the symmetry of the molecule. The Lewis structure most closely resembling reality consists of two resonance structures.
Sulfur dioxide SO 2 Lewis Structure Hybridization. This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. We start with a valid Lewis.
Three stable resonance structures can. HNO3 NO2 H2SO4 HNO3 NO2 H2SO4 asked Aug 22 2018 in Chemistry by Sagarmatha. It discusses the molecular geometry bond angle.
We start with a valid Lewis structure and then follow these general rules. The actual structure of SO2 is a resonance hybrid of all three structures. Drawing correct lewis structure is important to draw resonance structures correctly.
But when I made the Lewis Structure for SO2 its stablest form was the S with double bonds to each O and one lone pair of electrons and the two O had two sets of lone pairs With this the formal charge was all zero and there wasnt any resonance as both the oxygen had double bonds so Im confused how. Resonance structures should have the same number of electrons do not add or subtract any electrons. The oxygens share the negative charge with each other stabilizing it and reducing the charge on either atom.
In a UA session there was a problem where it asked for three resonance structures for SO2. Example with 3 resonance structures. Each of these isomers.
The hybridization of the structure must stay the same. Use the resonance structures to solve the problems below. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms.
We start with a valid Lewis structure and then follow these general rules. We say that these are resonance structures of SO2. The first one posted in the question and its mirror image.
Note that SO2 is a bit. The two bonds are identical. There is a central sulfur atom S with.
A step-by-step explanation of how to draw the CH3NO2 Lewis Dot StructureThere are several ways to draw the CH3NO2 Lewis structure. This is a correct Lewis structure. The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left and two lone pairs of electrons on that oxygen and the sulfur with a double bond to an oxygen on the right and two lone pairs of electrons on that oxygen.
Check the number of electrons by simply counting them. Drawing correct lewis structure is important to draw resonance structures correctly. Resonance Structures - Oneonta.
There are three resonance structures SO2 Sulfur dioxide. There are three resonance structures CO2 Carbon dioxide.

1 Which Of The Following Pairs Are Not Resonance Chegg Com

Resonance Structures Easy Hard Science

Resonance Structures For So2 Sulfur Dioxide Youtube
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2
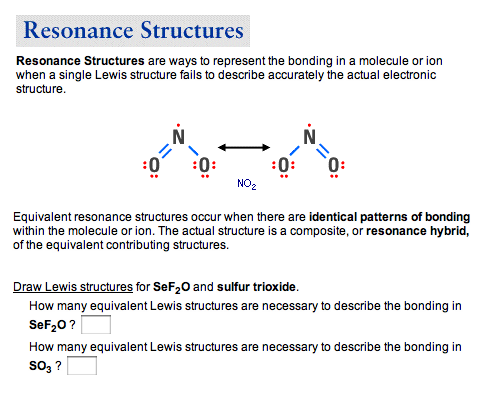
Resonance Structures Resonance Structures Are Ways Chegg Com

Draw The Lewis Structure Of Ch 3ncs Including All Resonance Forms Assign Formal Charges Do Not Include Resonance Arrows Study Com

Lewis Structure Of So2 Chemistry Stack Exchange
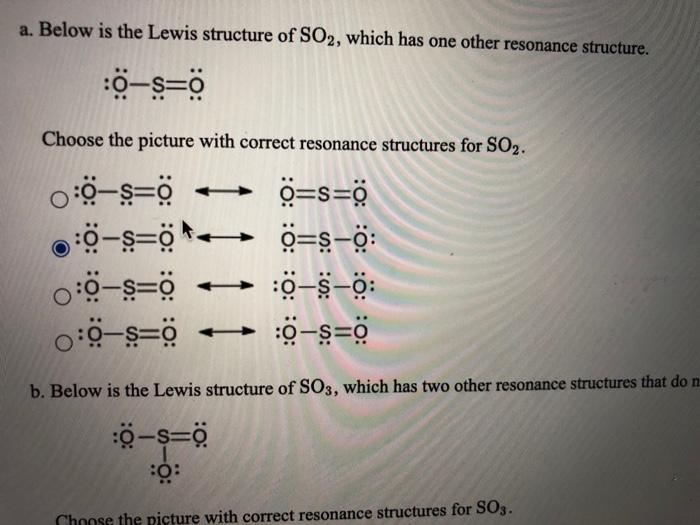
A Below Is The Lewis Structure Of So2 Which Has One Chegg Com

Resonance Structures For So2 Sulfur Dioxide Youtube
.jpg)
Lewis Structure For So32 Sulfite Ion Resonance Structures

Lewis Structure Sulfur Dioxide So2 Youtube
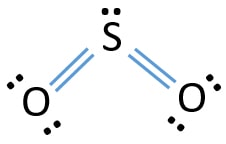
Sulfur Dioxide So2 Lewis Structure Hybridization

Formal Charges And Resonance Chemistry For Majors

Write Resonance Structure For So2 And Co2 Brainly In

Lewis Structure Of So2 Chemistry Stack Exchange
The Molecule So 2 Would Be Expected To Have A Two Identical Bonds Intermediate Between A Single And A Double Bond B Two Single Bonds C Two Double Bonds D One Single

The Correct Lewis Structure Of Sulfur Dioxide Youtube

Socl2 Lewis Structure How To Draw The Lewis Structure For Socl2 Youtube