N2h4 Lewis Structure Polar Or Nonpolar
It is very polar. If you look at the Lewis structure for C2H4 it appears to be a symmetrical molecule.

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep
N2H4 is polar in nature and dipole moment of 185 D.

N2h4 lewis structure polar or nonpolar. Electrons on the outer atoms are omitted for clarity. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Since both N N atom are identical we can just consider one N N atom which has 4 electron groups around it.
The hybridization of each nitrogen in the N2H4 molecule is Sp 3. Nitrogen trifluoride NF3 lewis dot structure molecular geometryshape polar or non-polar Bond angle hybridization. Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.
Another non polar molecule shown below is. Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. List molecules polar and non polar.
In this article we will discuss Nitrogen. Draw the Lewis structure of C₂H₄Cl₂ both Cl atoms on one C atom and then determine if the molecule is polar or nonpolar. N2H4 is a polar molecule because the unshared electron pairs of the nitrogen atoms create an area on the molecule that is more negative than the space around the hydrogen atoms.
IF3 Iodine trifluoride is Polar. Yes H2CS is a Polar Molecule. It is slightly soluble in water and very toxic by inhalation.
CF 2 H 2 e. N H 4 C l. CCl 2 F 2 d.
VSEPR predicts a tetrahedral geometry for the electron pairs. Also only one line of symmetry can be drawn through the N2H4 dash model. It is non-flammable with the chemical formula NF3.
Valence electrons bond pairs lone pairs sigma bonds pi bonds electron pair. Does n2h4 have a polar or nonpolar bond and ch4 is not a polar molecule. If there is only one line of symmetry the molecule is polar.
Total 2 lone pairs and 5 bonded pairs present in N2H4 lewis dot structure. C H 4 Medium. In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
Which molecule contains both polar and nonpolar covalent bonds. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. The formal charge on nitrogen in N2H4 is zero.
H 2 O m. Question Is N2H4 polar or nonpolar. Nitrogen trifluoride is a colorless gas with a smell like moldy.
N2H4 is a polar molecule because the unshared electron pairs of the nitrogen atoms create an area on the molecule that is more negative than the space around the hydrogen atoms. In chemistry polarity refers to the distribution of electric charge around atoms chemical groups or molecules. Notice that a tetrahedral molecule such as ceCCl_4 is nonpolar Figure PageIndex1.
CH 4 tetrahedral non-polar b. A non polar molecule is a molecule that has an even distribution of electrons. CH 2 O f.
Each nitrogen in N2H4 has four pairs of electrons around it a lone pair a single bond to the other nitrogen and a single bond to each of the hydrogens. Polar Nonpolar Ionic Chemical Bonds Lewis Structure. A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms togethers is called a n a.
Ill tell you the polar or nonpolar list below. The total valence electron available for the Hydrazine N2H4 lewis structure is 14. There will be two lone pairs on Sulfur.
Here is the Lewis structure S 2 lone pairs ll. Chemistry QA Library Draw the Lewis structure of C₂H₄Cl₂ both Cl atoms on one C atom and then determine if the molecule is polar or nonpolar. The N-N bond is non-polar but the N-H bonds are polar hydrogen has a lower electronegativity than nitrogen has.
So it is non polar. This lesson include different types of bonds such as polar nonpolar ionic chemical bonds. NCl 3 trigonal pyramidal polar c.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. N 2 O i. To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures.
Therefore its electron geometry is. H 2 O 2 D. Answer N2H4 Hydrazine is Polar What is polar and non-polar.
Nonpolar molecules also form when atoms sharing a Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Answer N2H4 Hydrazine is Polar What is polar and non-polar. If you draw the Lewis Structure you have the central atom of Carbon single bonding to two hydrogen and double bonding to sulfur.
Since N2O is linear and N2O is bent in shape so no need to check Electronegativity difference. The Lewis structure of N2H4 N 2 H 4 is shown below. The electrons involved in the formation of a chemical bond are called.
Valence electrons bond pairs lone pairs sigma bonds pi bonds electron pair geometry molecular shape polar or nonpolar valence electrons bond pairs lone pairs sigma bonds pi bonds electron pair geometry molecular shape polar or nonpolar Molecule water H20 Lewis Structure. CCl 2 F 2.

Lewis Structure Of Hydrazine Sigma Bonds Pi Bonds And Lone Pairs Youtube
Is Hydrazine An Organic Compound Quora
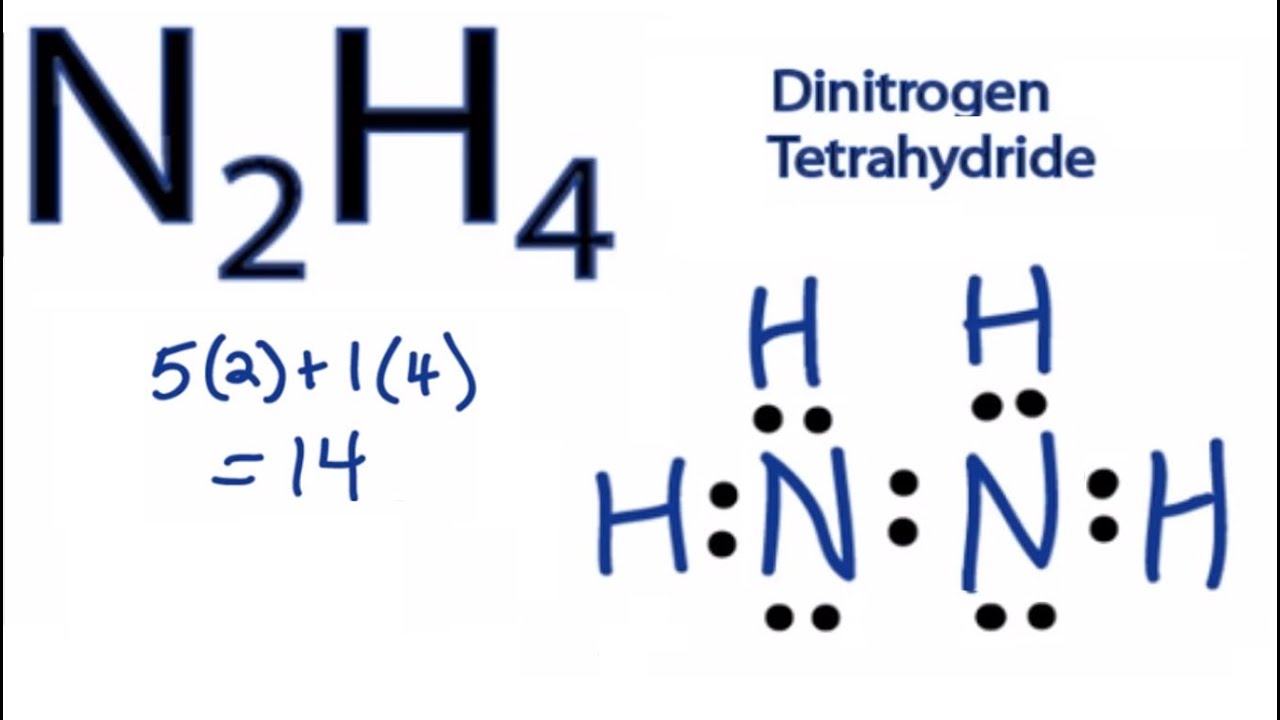
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
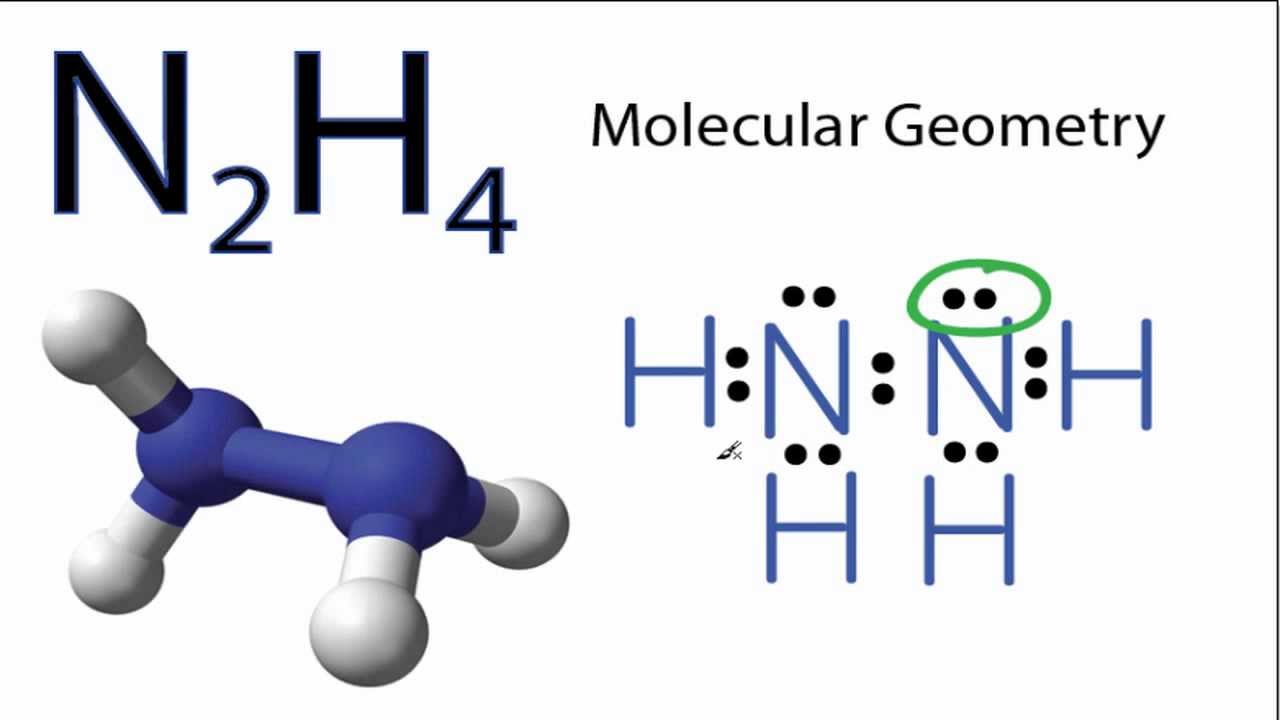
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
Does N2h4 Have A Polar Or Nonpolar Bond And Molecule Quora
Lewis Structures And Molecular Structure

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep

Hydrazine N2h4 Hydrazine Polar Molecule

Why Doesn T N2h4 Form A Dative Bond Quora

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
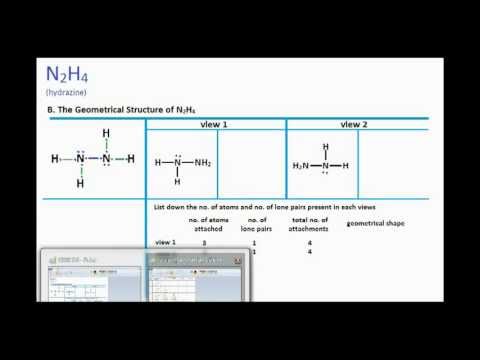
N2h4 Lewis Structure And Molecular Geometry Youtube

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep
Why Is Hydrazine N2h4 Polar It Seems To Me That The Sum Of The Left Side S Dipole Moments And The Right Side S Dipole Moments Are In Opposite Directions And Would Cancel Out

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist




