Ncl3 Electron Geometry
Total number of valence electrons of PCl3. View Ncl3 Molecular Geometry Gif.
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
Which of these isare polar molecules with a tetrahedral electron geometry.

Ncl3 electron geometry. A quick explanation of the molecular geometry of NCl3 Nitrogen trichloride including a description of the NCl3 bond anglesLooking at the NCl3 Lewis struct. A quick explanation of the molecular geometry of NCl3 Nitrogen trichloride including a description of the NCl3 bond anglesLooking at the NCl3 Lewis struct. Each chlorine atom.
Answered Jun 25 2017 by. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3.
The Correct Answer is. Check the link it is a sheet describing the different types of electron and molecular geometry. The electron-pair geometry is linear the molecular geometry is linear.
The IM was validated in terms of linearity R. The net dipole moment of Nitrogen trichloride is 06 D. The electron-domain geometry and molecular geometry of iodine.
Our sampling of the monitored swimming pool environments evidenced a mean NCl3 level 637-220 ugcu m higher than the recommended WHO value 500 ugcu m. Both are of different size and bond is more polar towards N as it has more electronegativity than Cl. This pair exerts repulsive forces on the bonding pairs of electrons.
Molecule NCl3 N C l 3 has nitrogen as the central atom. N atom is 3 electrons short from noble gas configuration while Cl atom is one electron short from the noble gas configuration. Ncl3 4 h2o nh4oh 3 hclo.
Thus it can donate electrons easily and therefore NCl3 is more basic than NF3. I and III c. I II and III d.
The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral. An explanation of the molecular geometry for the BCl3 ion Boron trichloride including a description of the BCl3 bond angles. There are three bond pairs and one lone pair of nitrogen.
A linear b trigonal planar c bent d tetrahedron e trigonal pyramid. Lewis structure of Nitrogen trichloride contains 1 lone pair and 3 bonded pairs. Phosphorus Trichloride PCl3 has a total of 26 valence electrons.
NCl3 has a central N atom with three N-Cl bonds So its probably clear that you need to look at the symmetry around the N-atom. Which is more basic nh3 or NCl3. There are three single bonds and one lone pair of electrons in NH3 molecule.
Ncl3 4 h2o nh4oh 3 hcloIts molecular geometry is bent. Use VSEPR theory to predict the molecular geometry of nitrogen trichloride NCl3. Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride NCl3.
Asked Sep 20 2016 in Chemistry by SarahC. 1 mole NCl3 120366g NCl3 6022 x 1023 molecules NCl3 82 x 1022 molecules NCl3 x 120366g NCl36022 x 1023 molecules NCl3 16g NCl3 rounded to 2 significant figures Electron geometry of NI3. A eg tetrahedral mg tetrahedral B eg linear mg trigonal planar C eg trigonal planar mg bent D eg linear mg linear E eg tetrahedral mg trigonal pyramidal.
The shape is distorted because of the lone pairs of electrons. Asked Jun 25 2017 in Chemistry by GeneXa. Thus their valencies are 3 and 1 respectively.
Determine the electron geometry eg and molecular geometry mg of NCl3. See full answer below. Nitrogen being pentavalent has 5 electrons in its outermost shell which are available for other atoms to bond.
5 73. View Ncl3 Electron And Molecular Geometry Background. Hence the geometry of the molecule of ncl3 is trigonal pyramidal.
So NCl3 and CCl3 both have backbonding present. Answered Sep 20 2016 by ThorXL. Which makes electron availability more on N in NCl3 than NF3.
NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a coloration proportional to the amount of NCl3 from the sampled indoor swimming pool air. Get Ncl3 Electron And Molecular Geometry Pictures. The electron geometry for the.
Hence the structure of the molecule of ncl3 is tetrahedral since a lone pair of electron can be considered to be a bond pair. Each chlorine atom sharing an electron pair with the nitrogen atom giving three bond pairs and six electron it has got nothing to do with the lone pairs. The electron-pair geometry is trigonal-planar the molecular geometry is trigonal-planar.
Valence electrons of Phosphorus Valence electrons of Chlorine.

Is Ncl3 Nitrogen Trichloride Polar Or Non Polar Youtube
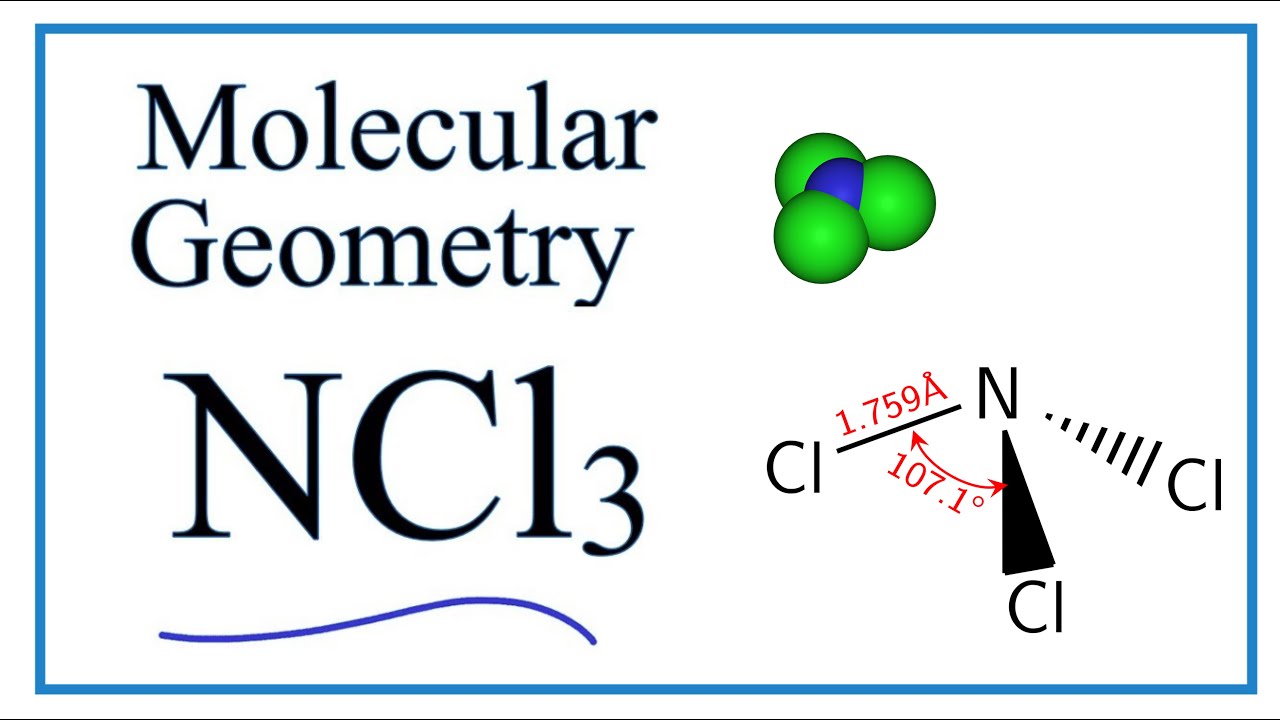
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Determine The Electron Geometry Eg And M Clutch Prep
What Is The Molecular Geometry Of Ncl3 Quora

Is Ncl3 Polar Or Nonpolar Quora

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube
File Ncl3 Dimensions Svg Wikimedia Commons

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
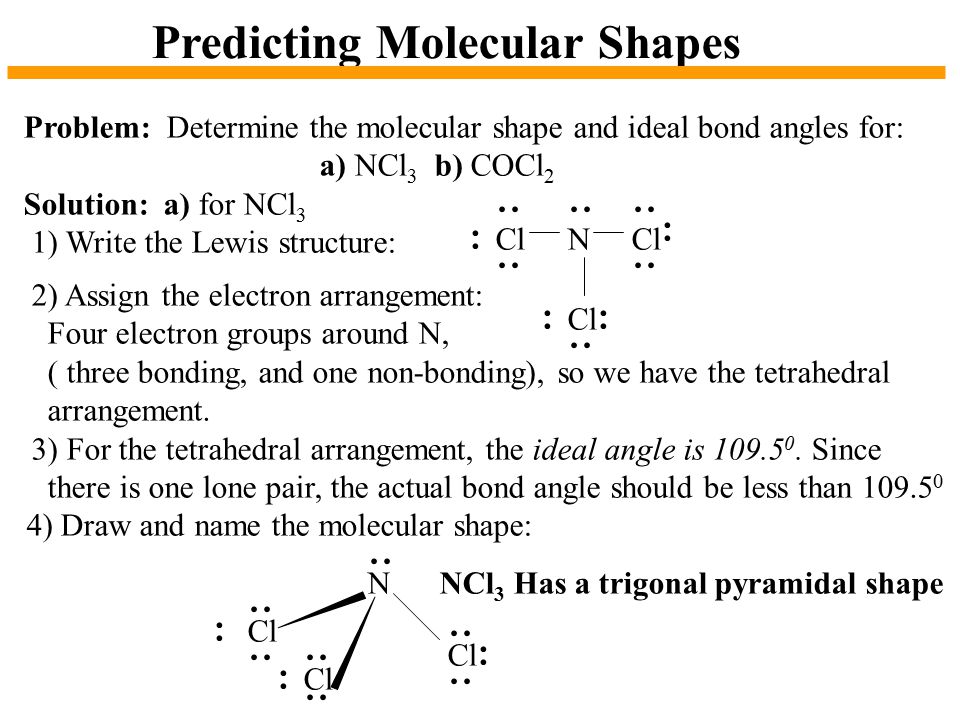
Chapter 10 The Shapes Of Molecules Ppt Video Online Download

The Lewis Structure For Ncl3 Is What Are Its Electron Pair Clutch Prep
Warm Up Draw Lewis Structures For The Compounds Below Cf4 Bf3 Co2 Ppt Download

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
Ncl3 Molecule Of The Month April 2017 Jsmol Version

Determine The Electron Geometry Eg And M Clutch Prep