Ncl3 Lewis Structure Bond Angle
Bond angle is the measuring parameter that measures the angle formed by the atoms which is connected with two covalent bonds in a molecule. Yes you are correct.
Wn Ncl2 Lewis Dot Structure Molecular Geometry Bond Angle
NF3 nitrogen trifluoride is very similar in structure to NCl3 and NH3 Lewis.
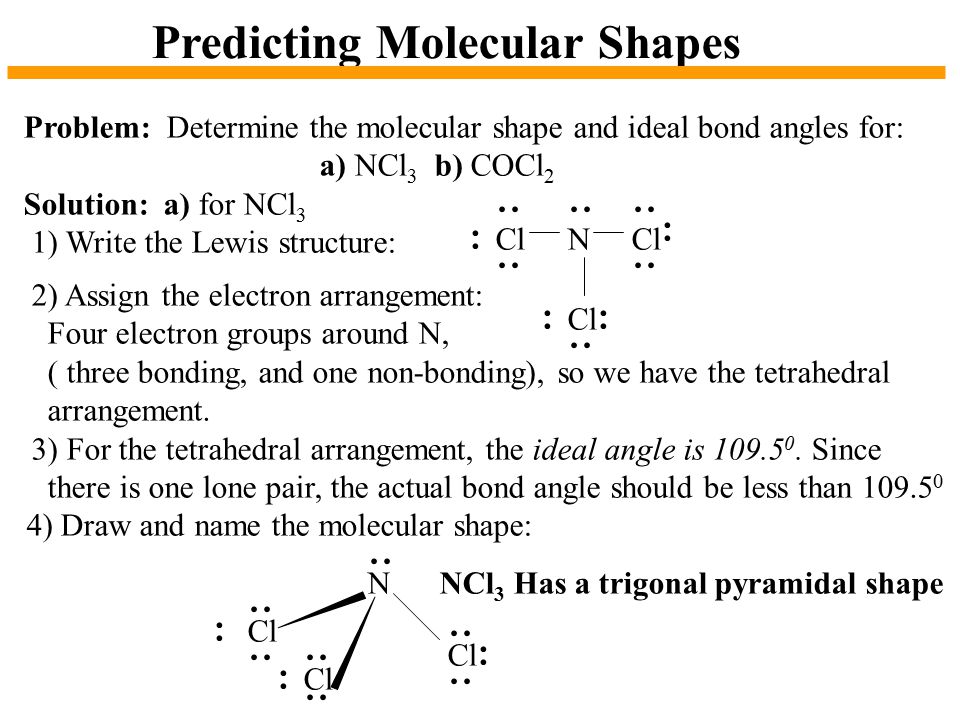
Ncl3 lewis structure bond angle. Hence the Geometry of the molecule of NCl3 is Trigonal pyramidal. This gives rise to a stable 120 degree angle between each of the bond-pair electrons. When we consider both lone pairs and bond pairs we are referring to the Structure of the molecule.
Quiz your students on AsF6- Lewis Dot Structure Molecular Geometry Bond Angle using our fun classroom quiz game Quizalize and personalize your teaching. To find the angle of the interior bond double click on one carbon atom drag to the vertex carbon atom and single click then drag to the 3rd carbon atom and double click. Draw Structure Name Molecular Geometry Of NCl3.
The lone pairs reduce the tetrahedral ideal angle of 1095 o between atoms by about two or four degrees depending on the individual atoms bonded together. Measure the angles formed in benzene. Sp 2 and Diamond.
These angle measurements can visualize the. But as there is one lone pair of electrons on the central phosphorus atom the bond angle will reduce from 109 degrees because of the repulsive forces of the lone pair. Molecule Lewis Structure Molecular Shape Bond Angle CS2 Linear 180 CH2O Trigonal Planar 120 H2Se Tetrahedral Bent.
Make a table that contains the Lewis structure molecular shape bond angle and type of hybrid for the following molecules. Sp 2 and sp 3. What is the Lewis structure of nf3.
Type below to check. Draw Structure Name Molecular Geometry Of NCl3. As per the molecular geometry of the molecule the bond angle of PCl3 should be 109 degrees.
This lone-pair of electrons push the N-Cl bond-pair further from the 120 degree angle giving rise to a lower bond angle. First click on spin off. Bond angles between 109-120.
Ii Shape Trigonal planar iii Bond angles Approximately 120. Learn how to draw a Lewis Structure and how to use it to assign a molecules shape geometryAre you taking a class with me. Is NCl3 polar or nonpolar.
Hence the Structure of the molecule of NCl3 is Tetrahedral since a lone pair of electron can be considered to be a bond pair. Electron pair geometry c. Being more electronegative Chlorine atom attracts the bonded electron pair slightly towards it and gains a partial negative charge and nitrogen atom gains partial positive charge.
Number of atoms each carbon. NCl3 takes this shape due to it following the AX3 E1 format. The Lewis structure NF3 has a total of 26 valence electrons.
Chemistry questions and answers. What is your value. CS2 CH2O H2Se CCl2F2 and NCl3.
Hydrogen escapes into the Lewis NF3 structure and all structures. Nitrogen N is the least electronegative element and is at the center of the Lewis structure of NF3. Number of electron groups b.
Draw Structure Name Molecular Geometry Of NCl3. This small difference between the predicted angle and the observed angle can be explained by proposing that the unshared pair of electrons on nitrogen repels the adjacent bonding pairs more strongly than the bonding pairs repel each other. So generally you are correct to assume that D is right.
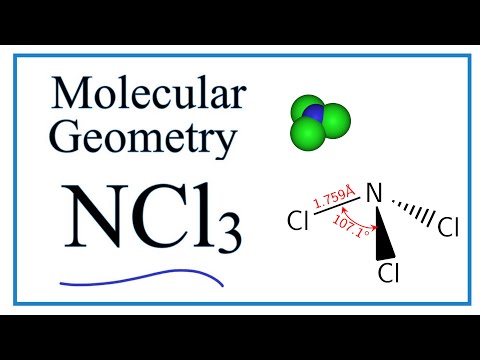
The bond angle of NCl3 is 1071 as it slightly decreases because of the lone pair present on nitrogen that creates repulsion between bond pairs and lone pair hence causes to decrease in angle. Our last example is NCL three which is try Gunnell para Middle with bonding angles of 1075 degrees and SP three hybrid orbitals so we can draw a few just him so Ill draw out CS two. If so feel free to rate me ath.
As a result the dipole moment of N-Cl comes out to be non zero. The observed bond angle is 1073. However the HNH bond angles are less than the ideal angle of 1095 because of LPBP repulsions Figure PageIndex3 and Figure PageIndex4.
The bond angle of Cl-N-Cl is around 1071 degrees and the bond length of N-Cl is 1759 A. Iv Polarity polar. Lone Pairs Of Electrons On Central Atom.
Ii Shape square planar iii Bond angles 90 iv Polarity nonpolar f HBF_2 i Lewis structure from wwwuwplattedu This is an AX_3 structure. The bond angles are slightly less than 120 slightly less than 1095 and 180 respectively. In NCl3 N has one lone-pair of electrons.
Draw the Lewis structure for NCl3 and provide the following information. Lone Pairs Of Electrons On Central Atom Bond Angle.

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

File Ncl3 Dimensions Svg Wikimedia Commons

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube
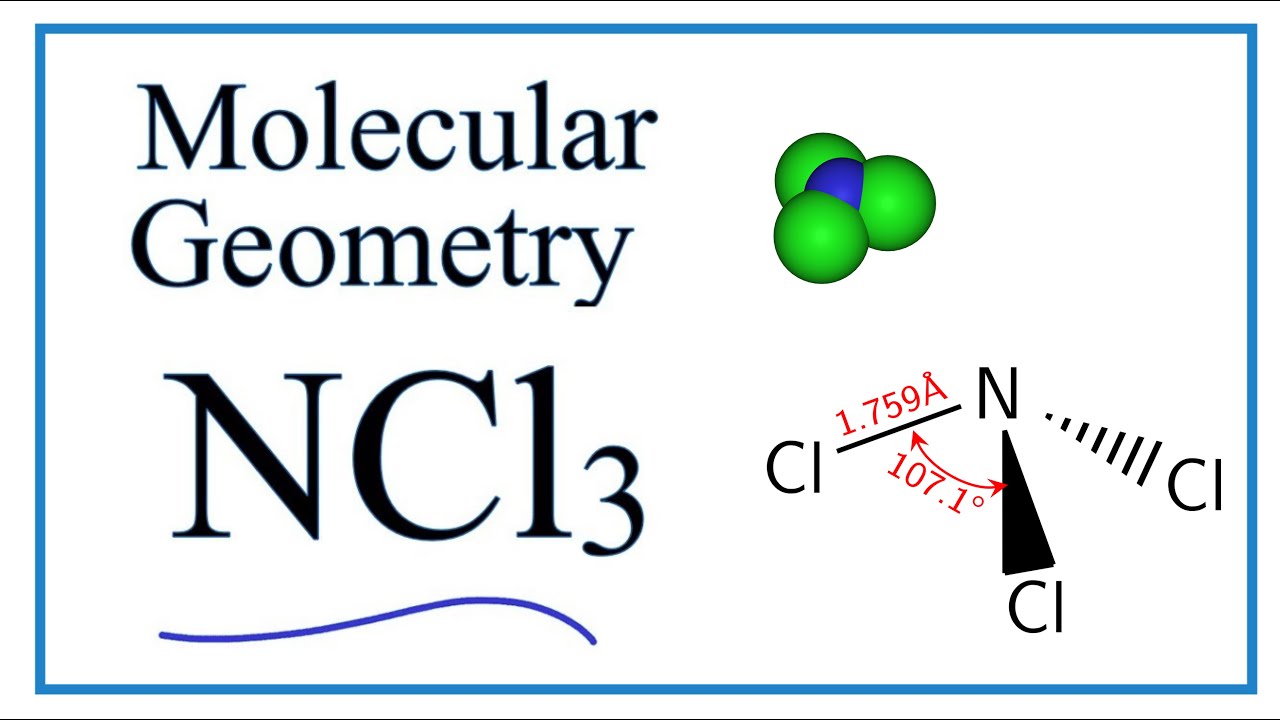
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep
What Is The Molecular Geometry Of Ncl3 Quora

Draw The Lewis Structure For Ncl3 And Provide The Following Information A Number Of Electron Groups B Electron Pair Geometry C Bond Angle D Number Of Bonded Electrons E Molecular Geometry F
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora
Chapter 10 The Shapes Of Molecules Ppt Video Online Download
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora

What Is The Electron Domain Geometry Aroun Clutch Prep

Nitrogen Trichloride Ncl3 Lewis Dot Structure Youtube
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

Is Ncl3 Polar Or Nonpolar Techiescientist

Hybridization For Ncl3 Description Of Hybrid Orbitals For Nitrogen Youtube

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube