Nho2 Lewis Structure
Watch out a lot more about it. Gives us a total of 18 valence electrons for the NO2- Lewis structure.

Hno2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Its not common to have an odd number of valence electrons in a Lewis structure.

Nho2 lewis structure. The HNO 2 Lewis structure is easier to think of if you consider it NO 2 with an H bonded to one of the oxygen atoms. Heshan Nipuna last update. With NH 2 OH youll need to attach the -OH group to the central Carbon.
It is a conjugate acid of a nitrite. The Lewis structure of SO2Cl2 is constructed as follows. For Nitrogen we have 5 valence electrons.
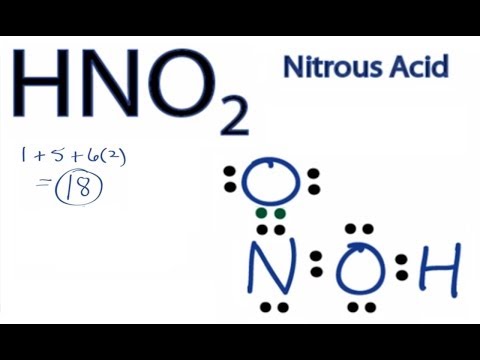
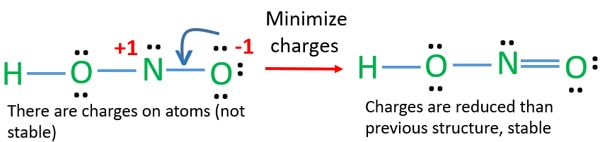
Now you need to calculate the number of Pi bonds using the lewis structure and octet rules. However in reality hydrogen atom is rather prone to migration and the second structure is not favorable. HNO2 Lewis Structure Molecular Geometry Hybridization and Polarity HNO2 or Nitrous Acid comes under the category of monoprotic acids acids that donate one proton during dissociation.
6 for Oxygen but we have two Oxygens so well multiply that by two. Because of this well try to get as close to an octet as we can on the central Nitrogen N atom. For the NO2- Lewis structure calculate the total number of valence electrons for the NO2- molecule.
Do this by the periodic group numbers for each atom in the structure and adjusting for and. Let us look at the periodic table. Single bond the two Cl atoms 180 degrees away from each other to the S atom.
This is the NO2- Lewis structure. Hybridization is a quantum phenomenon where energy is redistributed from atomic orbitals to form new hybrid orbitals that have equivalent energy. There are a total of 18 valence electrons for the Lewis structure for NO2-.
The NO2 Lewis structure has a total of 17 valence electrons. NO2 lewis Structure Nitronium ion Nitronium ion NO2 is a one type of cation. Helo reders welcome to another fresh article on textilesgreenin today we will discuss about nitronium ion and NO2 lewis structure.
After determining how many valence electrons there are in NO2- place them around the central atom to complete the octets. I have made this guide to help you out. Note that Hydrogen atoms always go on the outside of a Lewis structure.
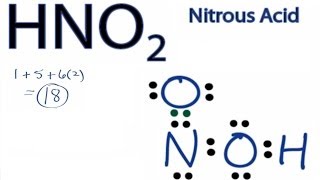
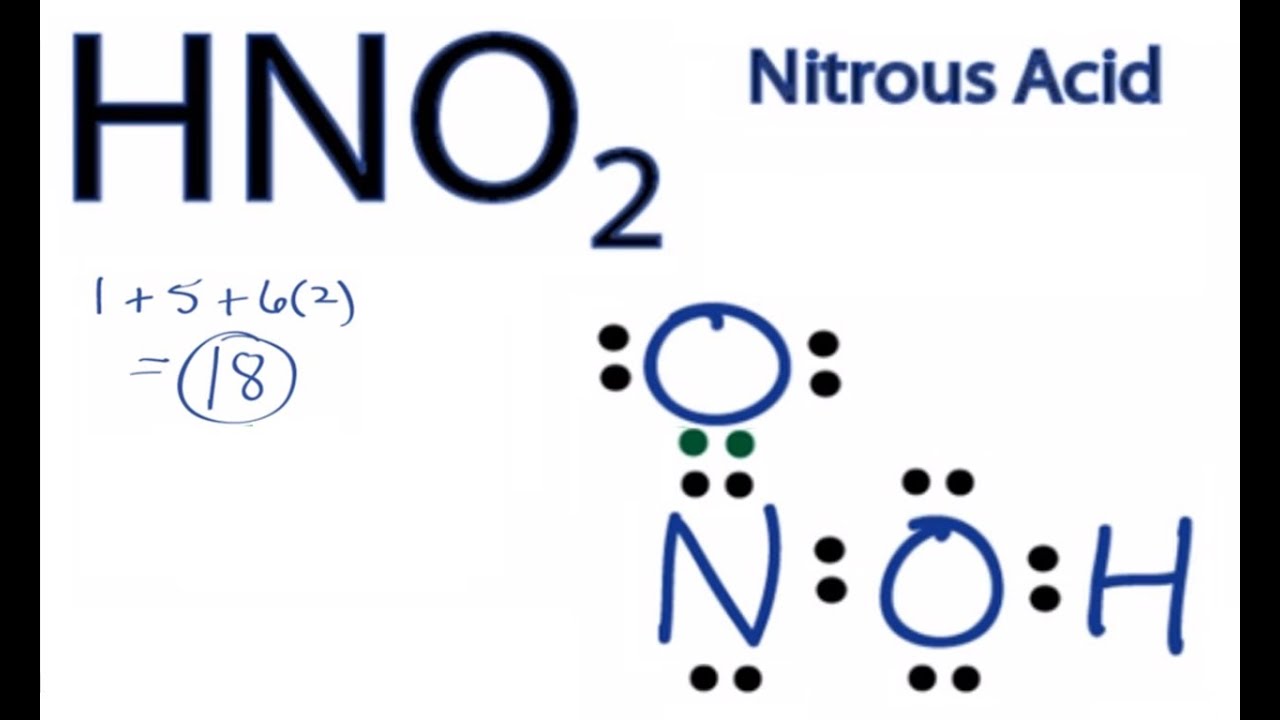
Nitrogen atom is the center atom in HNO 2. It is on the World Health Organizations List of Essential Medicines a list of the most important medications needed in a. Therefore P 6n 2 V 6 3 2 16 4.
Place the S atom at the center. In HNO 2 Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. Lewis Structure is otherwise called electron-dot structure.
Therefore the Lewis Structure for the HNO 2 is represented as follows. Plus one for this valence electron up here. Double bond the S atom to 2 O atoms 180 degrees from each other.
This gives an idea about the chemical bonding number of lone pairs the number of single bonds or double bonds number. Nitrogen dioxide does not have a single Lewis structure on account of its relatively strange electron configuration. In the lewis structure of NO 2 there is a unpaired electrons on nitrogen atom.
Nitrous acid as sodium nitrite is used as part of an intravenous mixture with sodium thiosulfate to treat cyanide poisoning. There are no charges on atoms and one double bond exists between nitrogen and one oxygen atom in the lewis structure of nitrous acid. A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure Nitrite ionFor the NO2 - structure use the periodic table to find the total number.
The location of the double bond changes over time meaning that at any point either of the oxygen atoms could have a double bond with the nitrogen atom. Nitrogen belongs to group 15 or group 5 and has an atomic number of 7 therefore has a valency of 5. There are one CO bond one C-O bond and one O-H in HNO 2 lewis structure.
Check the formal charges to be sure that each atom has a. So for a compound with composition N H X 3 O the correct structure would be H X 2 N O H. If I talk about the NO2 it consists of three atoms Where V 6 5 6 1 which becomes 16.
Do only the step asked for in each part and then click Check-dont work ahead to solve the final structure Step 1 Count valence electrons in the molecule or ion. Technically both the structures H X 2 N O H and H X 3 N O may exist. Lewis Structure of NO2 A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen.
Lewis structure of nitrous acid. Determine oxidation number of nitrogen in NO 2 NO 2- lewis structure N 2 O lewis structure resonance structures N 2 O 5 resonance structures Resonance structures examples Nitrogen dioxide acidity. As such nitrogen dioxide is represented by the resonance Lewis structure.
But in the NO 2- lewis structure there is lone pair on nitrogen atom. Nitrogen N goes at the center of the NH 2 OH since it is the least electronegative. Nitrous acid is a nitrogen oxoacid.
Create the Lewis structure of NO2 NOTE. This will mean that it will only have 7 valence electrons. It is a weak acid and exists only in solution form in the form of nitrite salts NO2-.
The structure of second type is stable for compound N O F X 3 and may be observed for amine oxides like C X 2 H X. There are a total of 14 valence electrons in the NH 2 OH Lewis structure. It is created by removel of electrons.
The formal charges being 0 for all of the atoms in the HNO 2 molecule tells us that the electron arrangement shown above is stable.

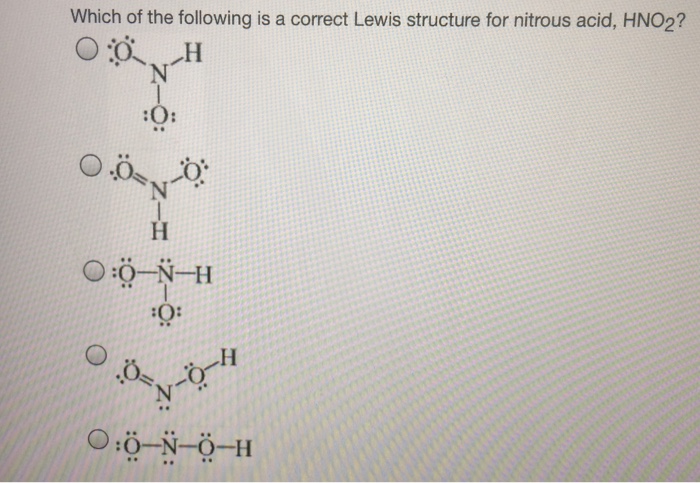
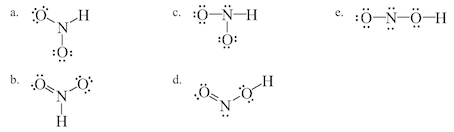
Oneclass Below Are Two Different Lewis Structures For Nitrous Acid Hno2 Which Is The Better Lewis
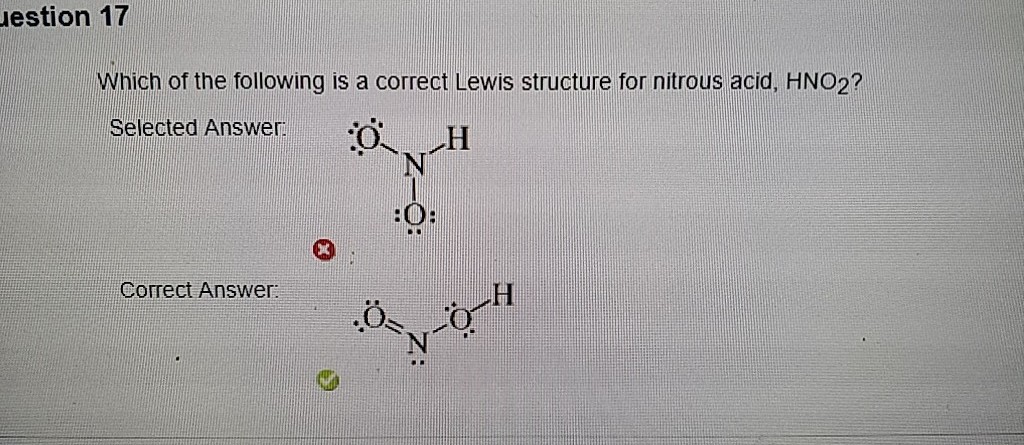
Estion 17 Which Of The Following Is A Correct Lewis Chegg Com

Oneclass Below Are Two Different Lewis Structures For Nitrous Acid Hno2 Which Is The Better Lewis
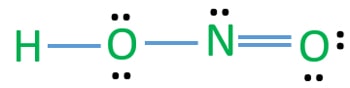
Hno2 Nitrous Acid Lewis Structure

Why Does Hno2 Not Have Resonance Chemistry Stack Exchange

Draw The Lewis Dot Structure Of Hno2 Brainly In

Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube

Nitrous Acid Structure Properties And Uses Of Hno2
Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube
File Nitrous Acid Svg Wikipedia
Which Of The Following Is A Correct Lewis Structure F
Solved 16 Consider Nitrous Acid Hno2 Hono A How Do I Write A Lewis Structure B What Are The Electron Pair And Molecular Geometries Of The Course Hero

Hno2 Lewis Structure Nitrous Acid Youtube
Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube
Which Of The Following Is A Correct Lewis Structure Chegg Com

Hno2 Lewis Structure Nitrous Acid Youtube
Hno2 Nitrous Acid Lewis Structure

Hno2 Lewis Structure How To Draw The Lewis Structure For Nitrous Acid Youtube