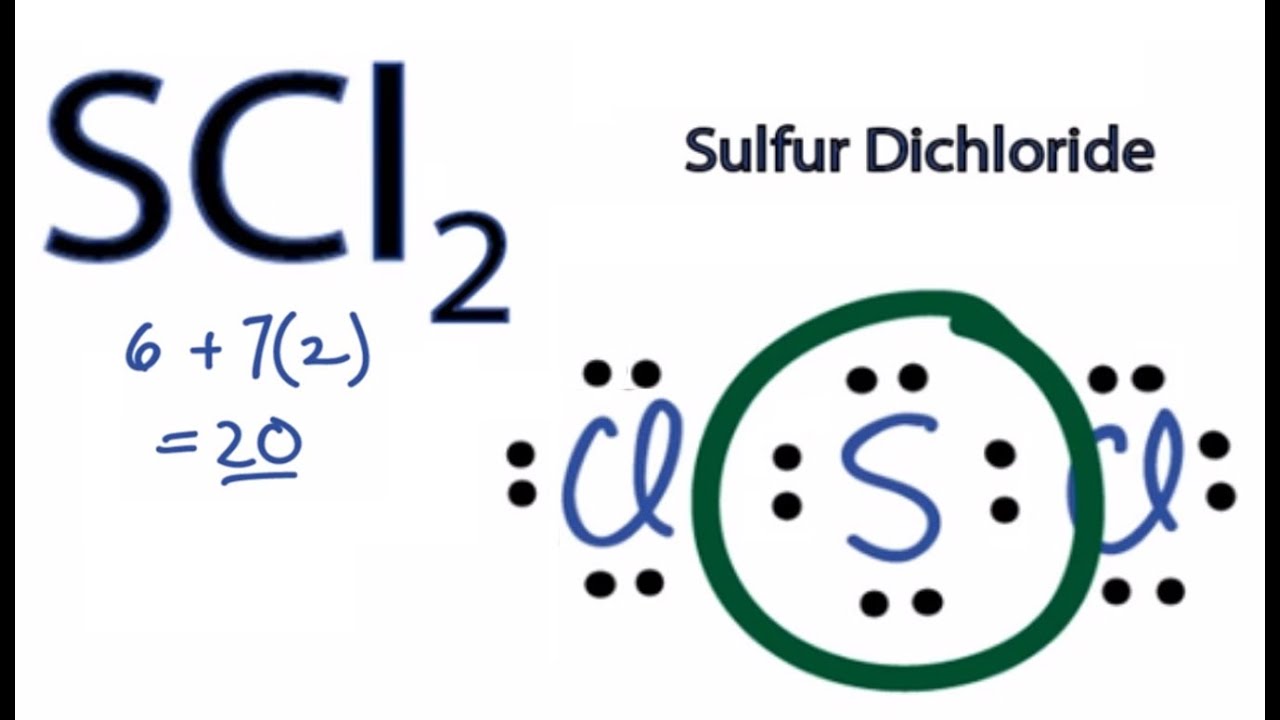
Scl2 2- Lewis Structure
The molecular shape of Cl2 is linear. SBr2 Lewis dot structure.

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
Van Nostrand Reinhold Co 1987 p.

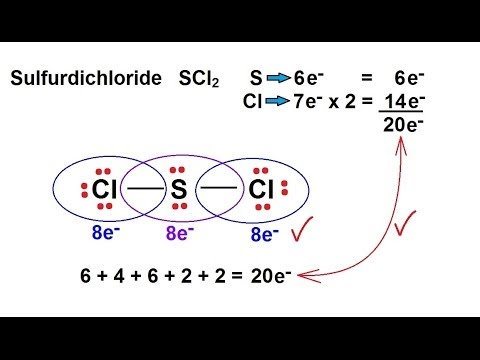
Scl2 2- lewis structure. First draw the Lewis dot structure. In the CH2Cl2 Lewis structure diagram the carbon atom can be the center atom of the molecule. SCl2 lewis structure contains one sulfur and two chlorine atom.
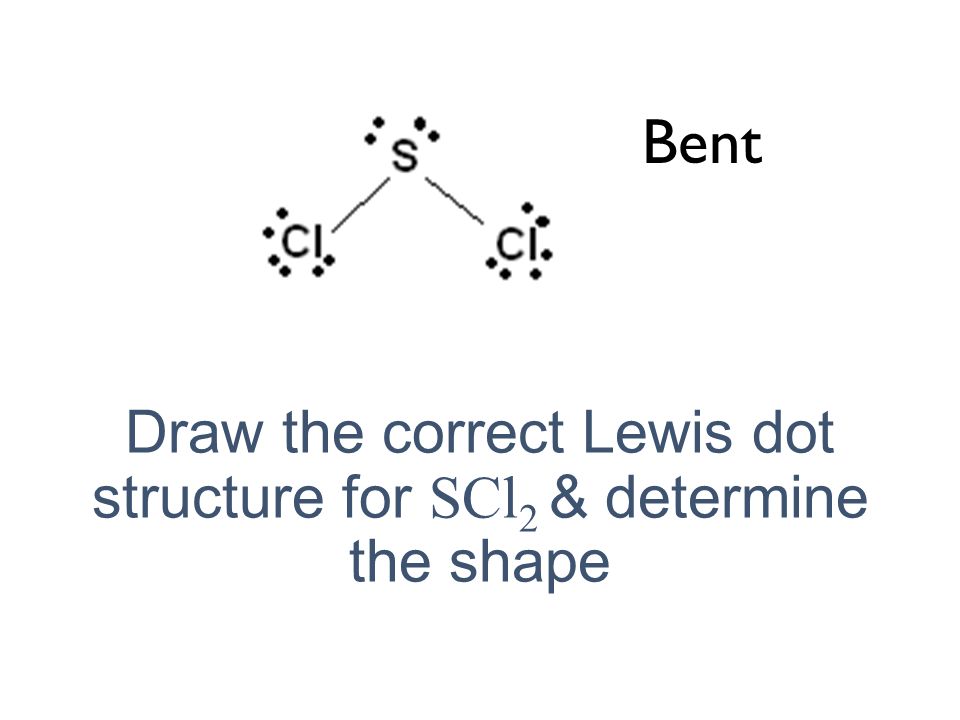
To draw the SCl2 Lewis structure follow the below instructions. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. Sulfur Fluoride is a highly unstable inorganic compound.
The Lewis Structure for SCl2 is given. Select all that apply. It might appear from the two dimensional drawing that the dipoles of the two S-Cl bonds should cancel one another to yield a nonpolar molecule.
The total valence electron available for drawing the Cl2 lewis structure is 14. The formal charge of Chlorine in the Cl2 lewis dot structure is zero. The central atom electron geometry is tetrahedral.
ES LES 12BE. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. Stabilized pure sulfur dichloride should contain minimum 98 SCl2.
The hybridization of each chlorine atom in Cl2 is Sp³. The molecular geometry shape is linear. According to Lewis structure SOCl 2 has three atoms that are attached to central Sulfur that include two Chloride and Oxygen.
First of all find out the total number of valence electrons in the C2H4 using the periodic table. The formal charge on the sulfur atom of SBr2 molecule V. It wont allow me to upload images.
None of these require pi-bonding which is the method of formation for double and triple bonds. Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70. Draw the Lewis structure for SCl 2 and use it to identify the correct statements that follow.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Formal charge on hydrogen atom of CH3I molecule 1- 0-22 0. ΔH 582 kJmol S 2 Cl 2 Cl 2 2 SCl 2.
What is the Lewis structure for SCl2. The molecule is nonpolar. The process occurs in a series of steps some of which are.
The product contains about 72 - 80 SCl2 residual S2Cl2 and Cl2. I cant draw it but each atom has 6 electrons around it. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol.
With a molar mass of 70062 gmol this compound is made up of one Sulfur atom and two Fluoride atoms. The addition of Cl 2 to S 2 Cl 2 has been proposed to proceed via a mixed valence intermediate Cl 3 S-SCl. SCl2 Lewis Structure Sulfur is the least electronegative and therefore is placed in the center of the skeletal structure.
There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure. As a result central carbon in the CH2Cl2 Lewis structure with all two chlorine and two hydrogens arranged in the tetrahedral geometry. The structure has one lone pair of electrons on the central atom.
SCl 2 is produced by the chlorination of either elemental sulfur or disulfur dichloride. ΔH 406 kJmol. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride.
Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewiss diagram and chlorine is spaced evenly around it. Thus the hybridization of SCl2 is sp3. Cl2 is non-polar in nature because of no dipole moment present in it.
Add valence electrons around the chlorine atom and add valence hydrogen atom as given in the figure. Use your molecular models to explain why this molecule is polar. In SCl2 the sulfur is in group 6 or 16 in.
SF2 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. In the Lewis structure of SCl2 the use of a double bond is necessary. Excess electrons that form lone pairs are represented as pairs of dots and are.
To calculate the formal charge on the central sulfur atom of the SBr2 molecule by using the following formula. S 8 4 Cl 2 4 S 2 Cl 2. First the valence electrons are placed around the carbon atom.
We then line up the two Chlorine atoms on either side of the Sulfur atom. For the scl2 lewis structure use the periodic table to find the total number of valence electrons for the scl2 molecule. In this post we discussed the method to construct the CH3I Lewis structure.
The structure of thionyl chloride showing the trigonal pyramidal geometry is shown below. By signing up youll get thousands of step-by-step solutions to your homework questions.

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com
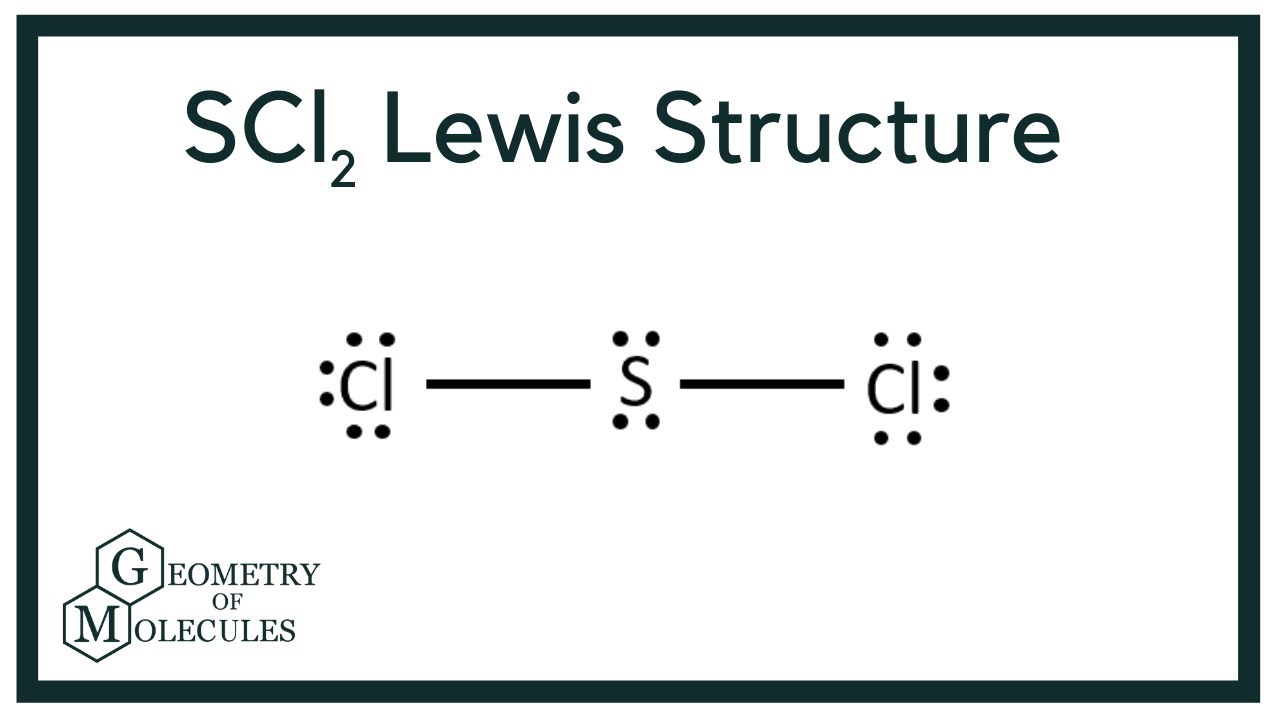
Scl2 Lewis Structure Sulfur Dichloride Youtube
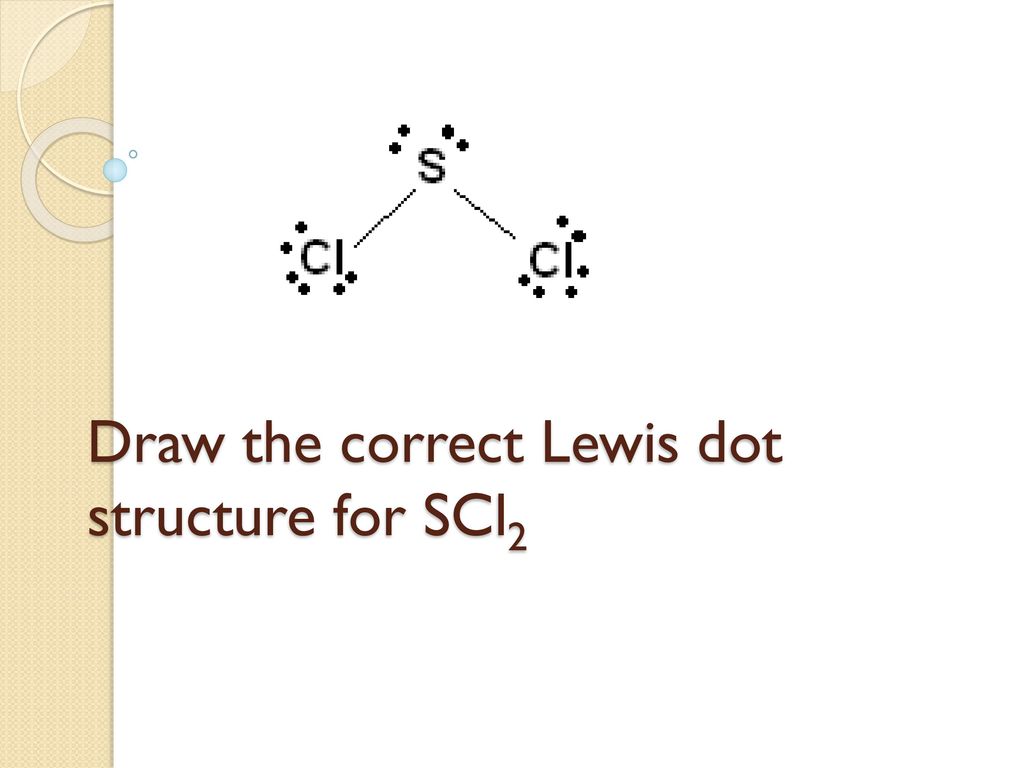
Types Of Bonding And Lewis Structures Ppt Download

Chem 110 Exam 2 Flashcards Quizlet

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds

Does Scl2 Have A Dipole Moment Clutch Prep
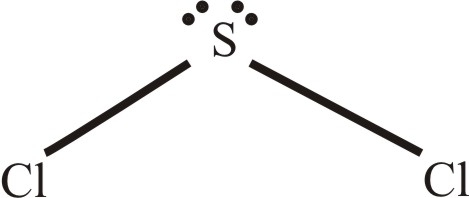
Solved Predict The Geometry Of Sulfur Dichloride Scl2 And The H Chegg Com

How To Draw Lewis Structure For Scl2 Drawing Easy

What Is The Name Of The Hybrid Orbitals Us Clutch Prep

Does Scl2 Have A Dipole Moment Clutch Prep

What Is The Lewis Structure For Scl2 Study Com
Lewis Structure Of Scl2 And Hybridization Of Scl2 Lewis Structures

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

The Lewis Diagram For Scl2 The Electron P Clutch Prep
Draw The Correct Lewis Dot Structure For Nacl Ppt Video Online Download
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity