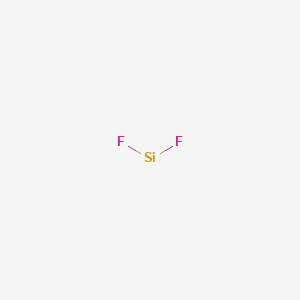Sif2 Lewis Structure
The Sulphur atom has sp3 Hybridization and the bond angle of F-S-F is 98 degrees. Elements in the first 2 periods of the Periodic Table do not have access to the d sublevel and must adhere to the octet or duet H and He rule.

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube
Get 1 free homework help.

Sif2 lewis structure. Hybridization in the Best Lewis Structure. Drawing SF2 Lewis Structure is very easy to by using the following method. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride.
In silicon it has four valence electrons Group number will be same as number of valence electrons and Fluorine atom have 7 valence electrons. Put Si in the middle least electronegative and put 2 H atoms and 2 F atoms attached to the Si. Be sure to put brackets and a 2- around the SiF 62- Lewis structure to show that it is an ion.
Four fluorine atoms have total 28 electrons. The initial concentrations of and are 140 and 0100 respectively. Best Lewis Structure The Lewis structure that is closest to your structure is determined.
Draw a Lewis structure for SiF5- and answer the following questions based on your drawing. The sulfur difluoride chemical formula is SF2. A bonding orbital for Si1-F2 with 19656 electrons __has 494 Si 1 character in a p204 d2 hybrid.
What is the Lewis structure for SiF 6 2-. The number of lone pairs The number of single bonds The number of double bonds 2. Here in this post we described step.
Sulfur Fluoride is a highly unstable inorganic compound. The Lewis structure for SF 2. SF2 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram.
For the central silicon atom. Obeys the octet rule B. The hybridization of the atoms in this idealized Lewis structure is given in the table below.
Get college assignment help at Only Professors Question What is the lewis dot structure for SiF2 Question A voltaic cell consists of a half-cell and a half-cell Question A voltaic cell consists of a half-cell and a half-cell at 25. With a molar mass of 70062 gmol this compound is made up of one Sulfur atom and two Fluoride atoms. Then put 3 lone pairs 6 electrons around each of the two F atoms.
That is the Lewis dot structure. SF 2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms. Laboratory Chemical Safety Summary LCSS Datasheet.
Fluorine is the most electronegative element on the periodic table and goes on the outside of the structure. Has an expanded octet. Has an incomplete octet.
The central silicon atom ____. There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent. For unlimited access to Homework Help a Homework subscription is required.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. There are a total of 48 valence electrons in SiF 62-. Silicon Si is the least electronegative and goes at the center of the Lewis structure.
In the SF 2 is Lewis structure Sulfur S which is the least electronegative and goes at the center of the Lewis structure. The VSEPR predicts the Octahedral shape. Ad_1 Get college assignment help at Students Paper Help Question What is the lewis dot structure for SiF2 Question A voltaic cell consists of a half-cell and a half-cell Question A voltaic cell consists of a half-cell and a half-cell at 25.
Now draw single bonds around the central atom connecting Fluorine atoms. Silicon Si is below Period Two on the periodic table and can hold more than 8 valence electrons. The hybridization of the atoms in this idealized Lewis structure is given in the table below.
Hybridization in the Best Lewis Structure. Question What is the lewis dot structure for SiF2 Read More. The initial concentrations of and are 140 and 0100 respectively.
Get college assignment help at Smashing Essays Question What is the lewis dot structure for SiF2 Question A voltaic cell consists of a half-cell and a half-cell The initial concentrations of and are 140 and 0100 respectively. A bonding orbital for Si1-F2 with 19907 electrons __has 1595 Si 1 character in a sp101 hybrid. Unlock all answers to this question.
SiF 62- is d 2 sp 3 hybridized and contains no lone pair and 6 bonding pairs of valence electrons around the Silicon.

Is Sf2 Polar Or Nonpolar Youtube

Wn Lewis Structure For Si2 Molecular Geometry And Hybridization
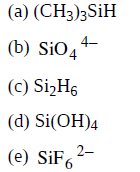
Solved Write A Lewis Structure For Each Of The Following Molecule Chegg Com
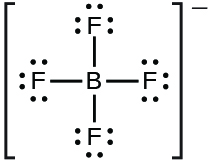
20 3 Structure And General Properties Of The Metalloids General Chemistry 1 2
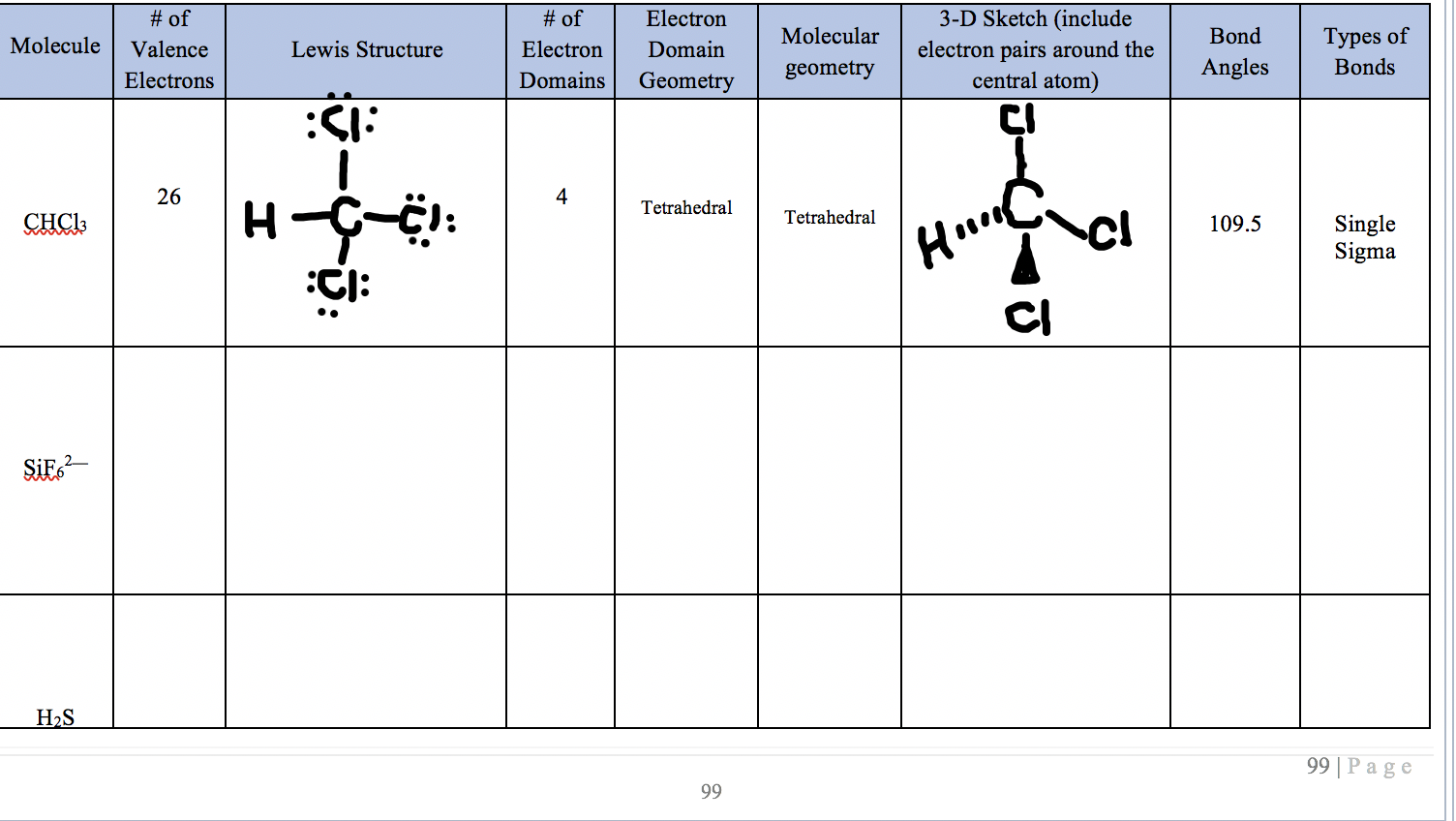
Of Molecule Lewis Structure Valence Electrons Of Chegg Com

How To Draw The Lewis Dot Structure For Si2 Sulfur Diiodide Youtube

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube

Sif2 Structure F2si Over 100 Million Chemical Compounds Mol Instincts

Sif2 Structure F2si Over 100 Million Chemical Compounds Mol Instincts
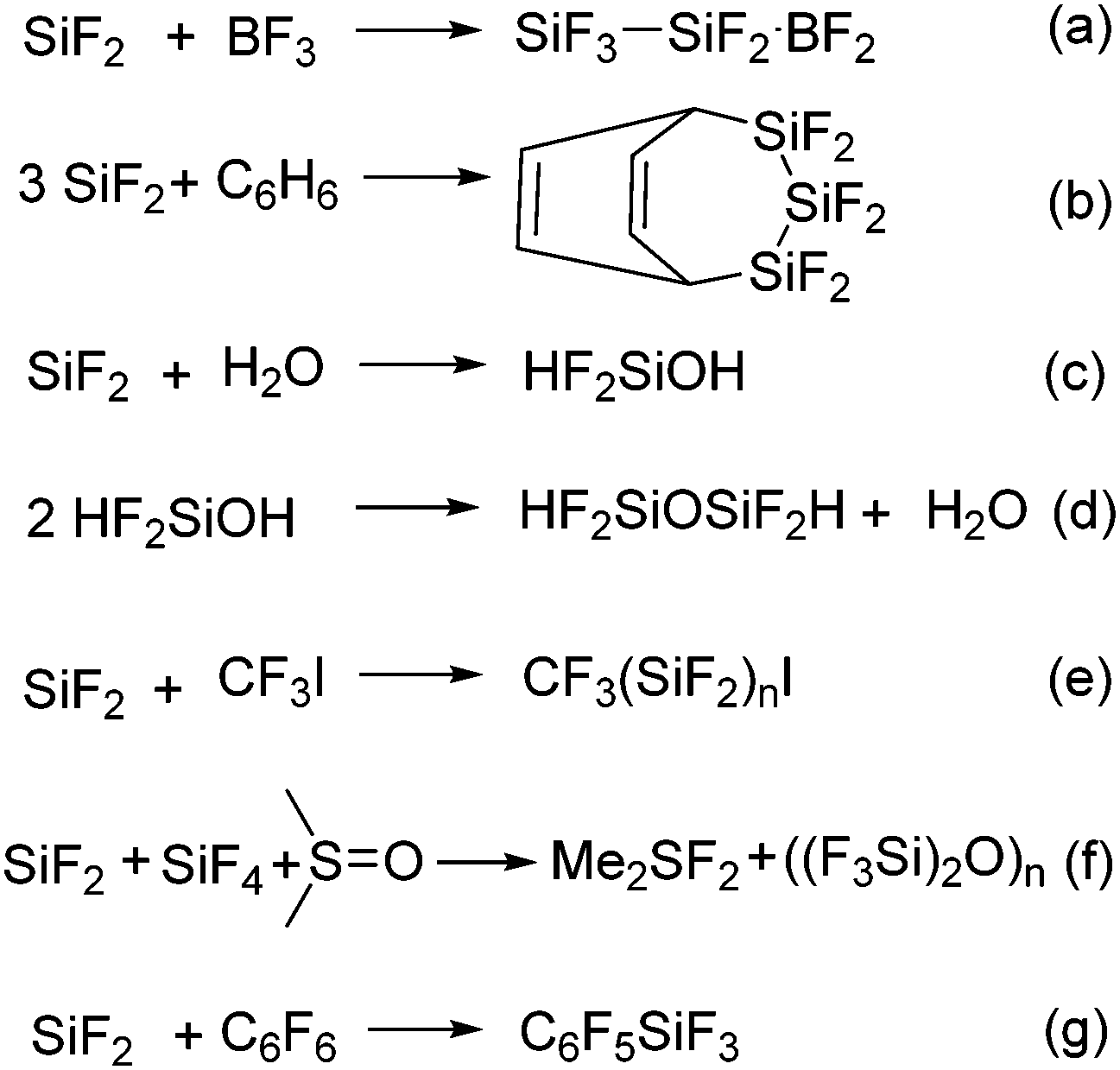
Silicon Fluorine Chemistry From The Preparation Of Sif 2 To C F Bond Activation Using Silylenes And Its Heavier Congeners Chemical Communications Rsc Publishing Doi 10 1039 C8cc01816b
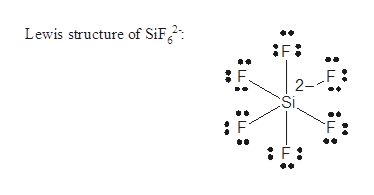
Answered Sif62 Nsf F2co Fno2 Deduce The Bartleby

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube
18 E Representative Metals Metalloids And Nonmetals Exercises Chemistry Libretexts