The Lewis Dot Structure For Water Shown Above Has How Many Lone-pair
After an atomic bomb has been dropped the damage continues to get worse. In a molecule central atom A has sp3 d hybridisation and is surrounded by some sigma bond pairs.
![]()
Find The Lewis Dot Structure Lds Of The Following Substance Na2so3 Study Com
The trial structure is You have eight valence electrons in your trial structure so it has the correct number of electrons.
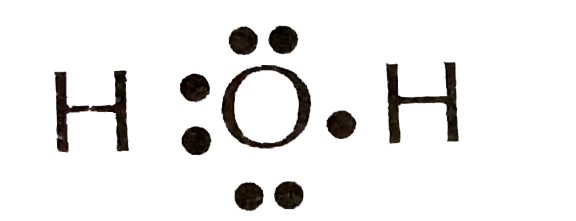
The lewis dot structure for water shown above has how many lone-pair. Count how many electrons from the. Physics 21062019 1330 QueenMiah16. Physics 21062019 2130 raieliz1414.
I have an account sign in. Iodine and three hydrogen atoms on the outside of the CH3I molecule to the central carbon atom in the middle. The outermost valence shell electrons of the SCl4 molecule are 10.
If the wheel is released from rest on the incline with the. 1 question The lewis dot structure for water shown above has how many lone-pair electron groups. Four electrons are shown as dots in the SCl4 chemical structure whereas four single bonds each contain two electrons.
Physics published 10. A valence electron is the outermost shell electrons around an atom. Four - the answers to brainsanswerscouk.
The lewis dot structure for water shown above how many bonded pair electron groups. The Lewis dot structure for water shown above has how many lone-pairElectron groups. The lewis dot structure for water shown above has how many lone-pair electron groups.
3 Get Other questions on the subject. The lewis electron-dot structure of n2 has ________ nonbonding electrons pairs ________ bonding electron pairs and a bond order of ________. O 6 4 - ½4 0 The Lewis structure of H₂O is.
In the Lewis structure for formic acid HCOOH how many bonding pairs and lone pairs of electrons ar. 1 on a question The Lewis dot structure for water shown above has how many lone-pair electron groups. Lewis dot Structure for CH3I generated from step-1 and step-2.
Count total valence electron in CBr4. Correct answer to the question The Lewis dot structure for water shown above has how many lone-pair electron groups. 1 Show answers Another question on Physics.
A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t. In this stage use four single bonds to connect all one. There will be two pairs for a total of 4.
The 10-kg double wheel with radius of gyration of 125mm about o is connected to the spring of stiffness k 600 nm by a cord which is wrapped securely around the inner hub. To get the valence electron of an atom look at its periodic group. Find another answers.
How many lone pairs of electrons will be shown around each hydrogen atom in the Lewis structure of water. Follow some steps for drawing the lewis dot structure of CBr4. H 1 - ½2 0.
Log In Join Now. How many lone pairs of electrons will be shown around each hydrogen atom in the Lewis structure of water. The Lewis dot structure for water shown above has how many lone-pair electron groups.
The formal charge on each atom is. Connect the exterior and core central atom of the CH3I molecule with four single bonds one C-I and three C-H. The Lewis dot structure for water shown above has how many lone-pair electron groups.
If your question is not fully disclosed then try using the search on the site and find other answers on the subject Physics. In the correct Lewis dot structure of CO_2 the total number of lone pairs are. Published 11052018 on subject Chemistry by Guest.
You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis diagram. The lewis dot structure for water shown above how many bonded pair electron groups.
The lewis dot structure for water shown above has how many lone-pair electron groups. Since we have to find the valence. Chlorine and one lone pair in the outermost valence shell.
I do not have an account I want to register. A step-by-step explanation of how to draw the H2O Lewis Dot Structure WaterFor the H2O structure use the periodic table to find the total number of valenc. The trial structure has the correct number of electrons.
There will be two pairs for a total of 4 electrons on the Oxygen atom in the water. Count how many outermost valence shell electrons have been used so far using the SCl4 Lewis structure.
How To Know Where To Put The Dots On A Lewis Structure Quora
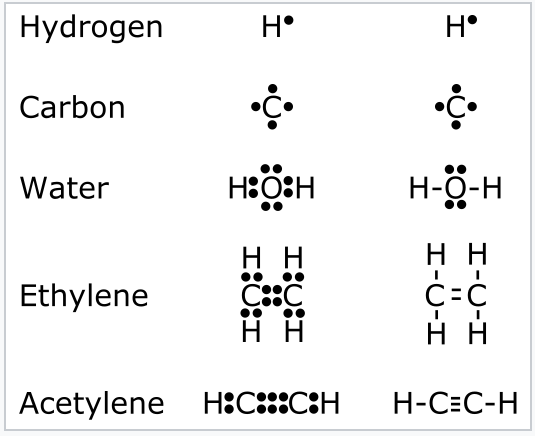
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts
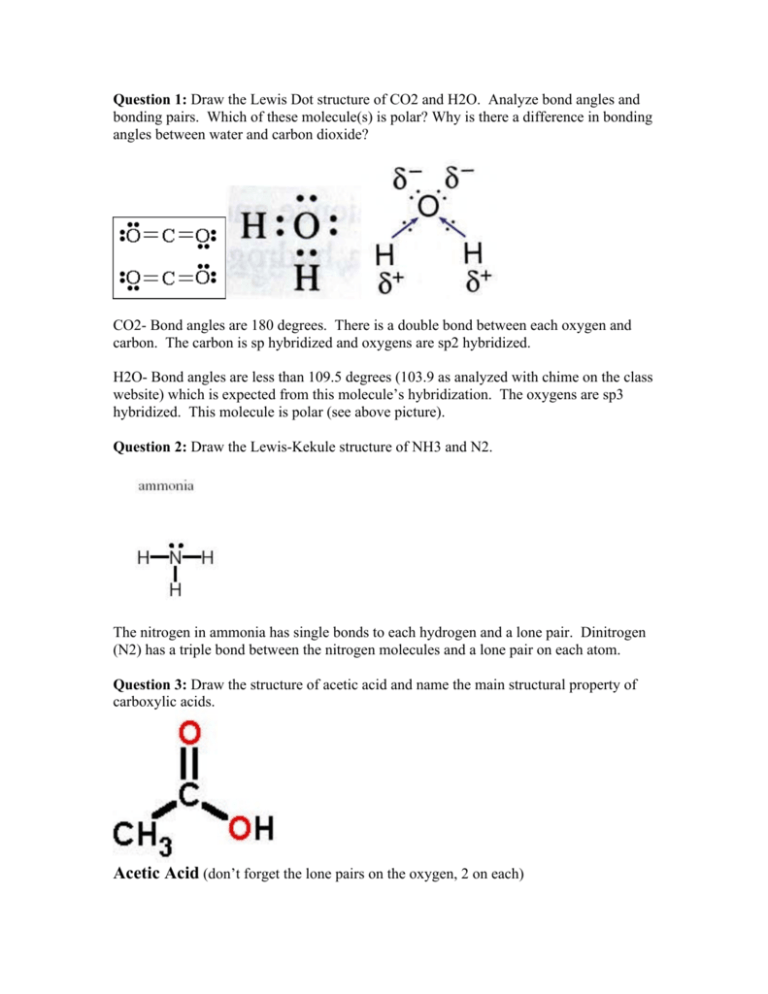
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

How To Draw The Lewis Dot Structure For Ch4 Methane Youtube
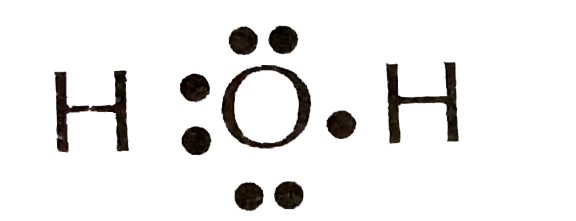
The Correct Electron Dot Structure Of Water Molecule Class 11 Chemistry Jee Main
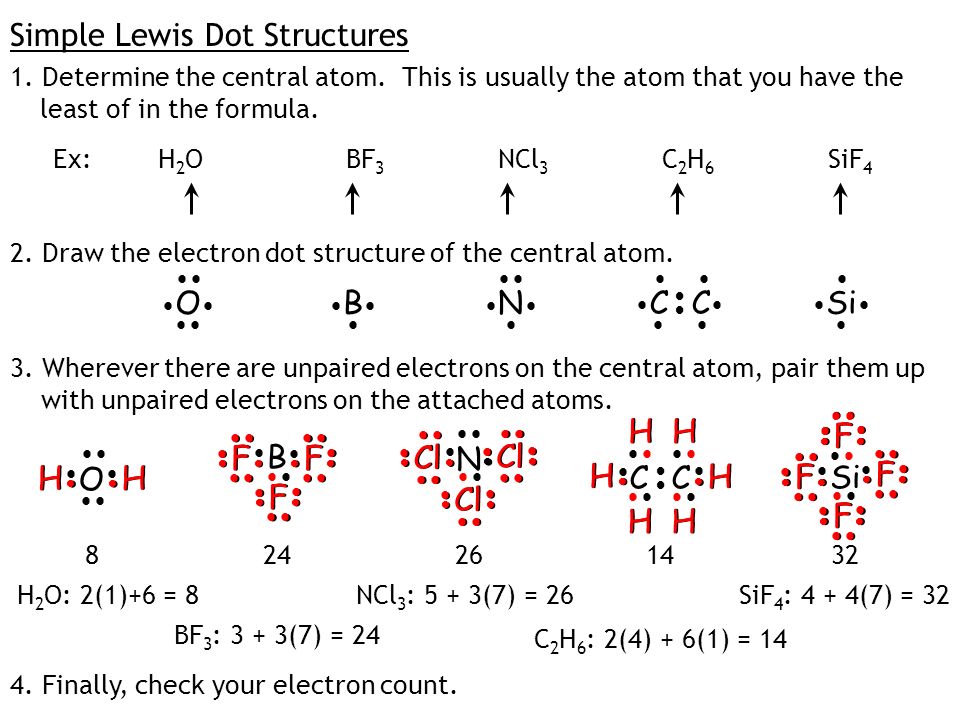
Lewis Dot Structures Electron Dot Structures Of Compounds Ppt Download

Oh Lewis Structure How To Draw The Lewis Dot Structure For The Hydroxide Ion Youtube

Co2 Lewis Structure Carbon Dioxide Youtube

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube
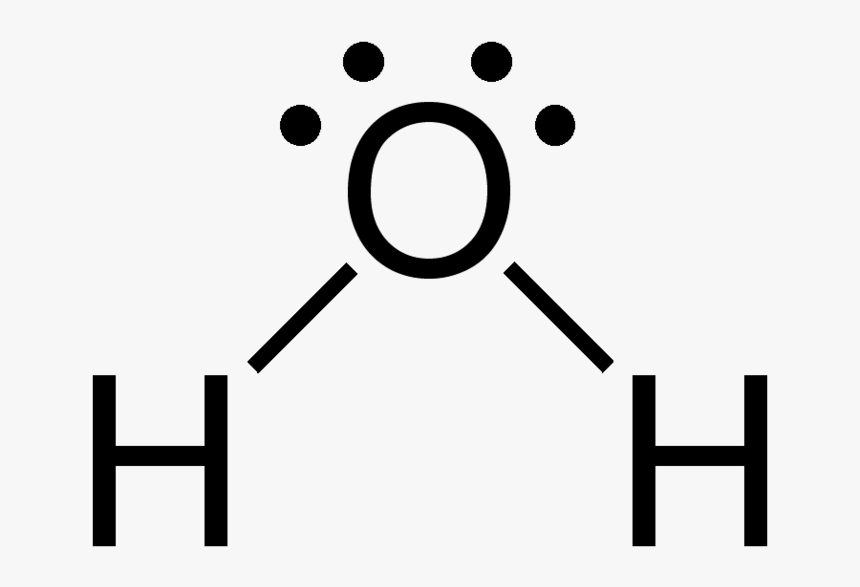
The Correct Electron Dot Structure Of Water Molecule Class 11 Chemistry Jee Main

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure For Nitrogen Atom N Youtube

Makethebrainhappy The Lewis Dot Structure For H2o

3 Ways To Draw Lewis Dot Structures Wikihow

Lecture 8 2 Lewis Dot Structures For Molecules

Hf Lewis Structure How To Draw The Dot Structure For Hf Youtube
How To Know Where To Put The Dots On A Lewis Structure Quora
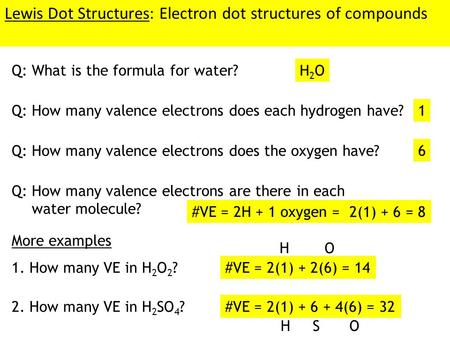
Lewis Dot Structures Electron Dot Structures Of Compounds Ppt Download
