Valence Electrons Of C2h4
Hydrogen is the least electronegative element here. Total valence electrons given by two carbon atoms 4 2 8 There are four hydrogen atoms in ethene molecule Therefore Total valence electrons given by hydrogen atoms 1 4 8.
Ethene C2h4 Lewis Structure Hybridization
Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr.
Valence electrons of c2h4. 34 36 and 38 valence electrons were studied. The hydrogen is in group 1 and has one valence electron. 2 Valence electrons in C 4 Valence electrons in H From the above calculation it is inferred that ethene has 16 valence electrons.
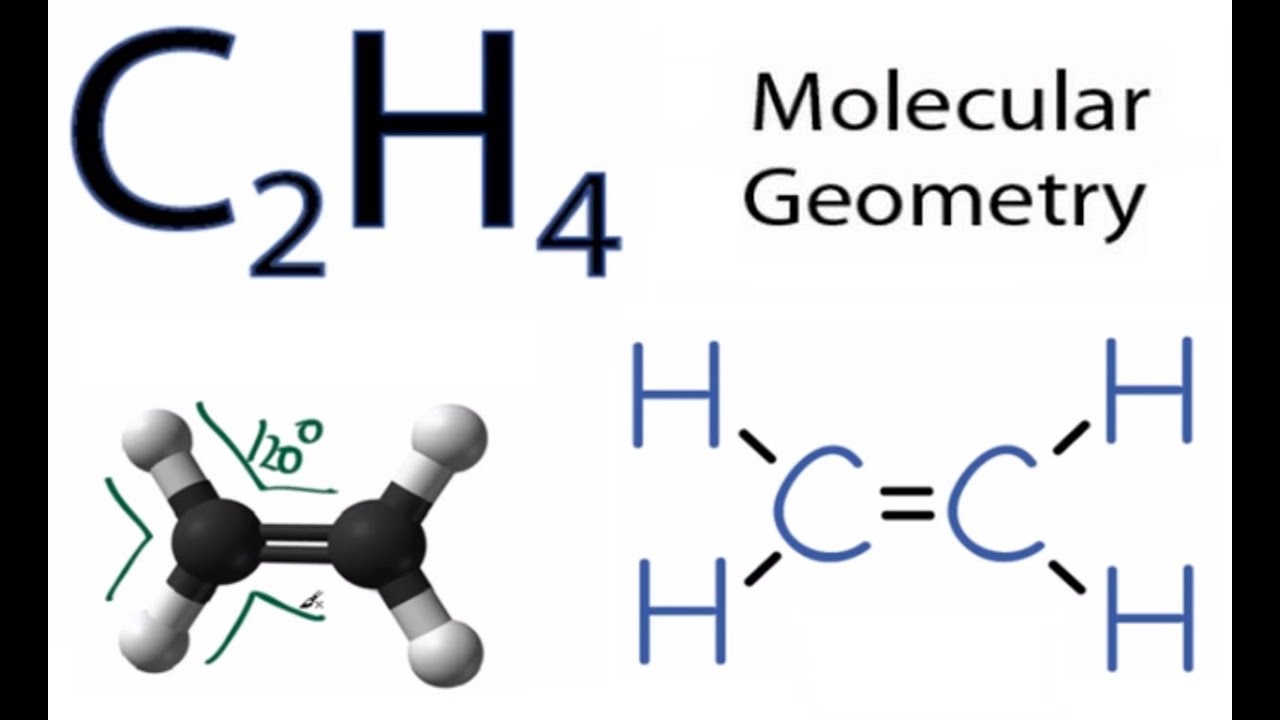
In a double bond two pairs of valence electrons are shared for a total of four valence electrons. Ethene from above the trigonal plane. Start by forming covalent bonds between the Carbon and Hydrogen atoms.
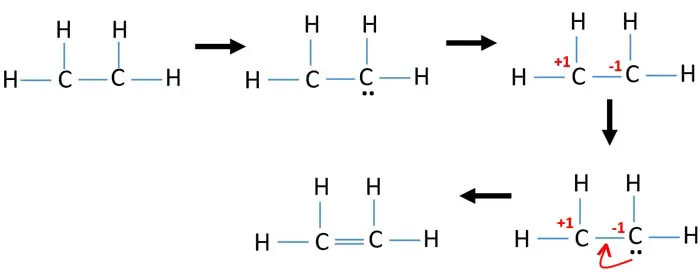
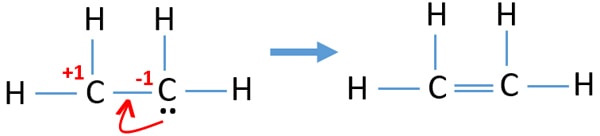
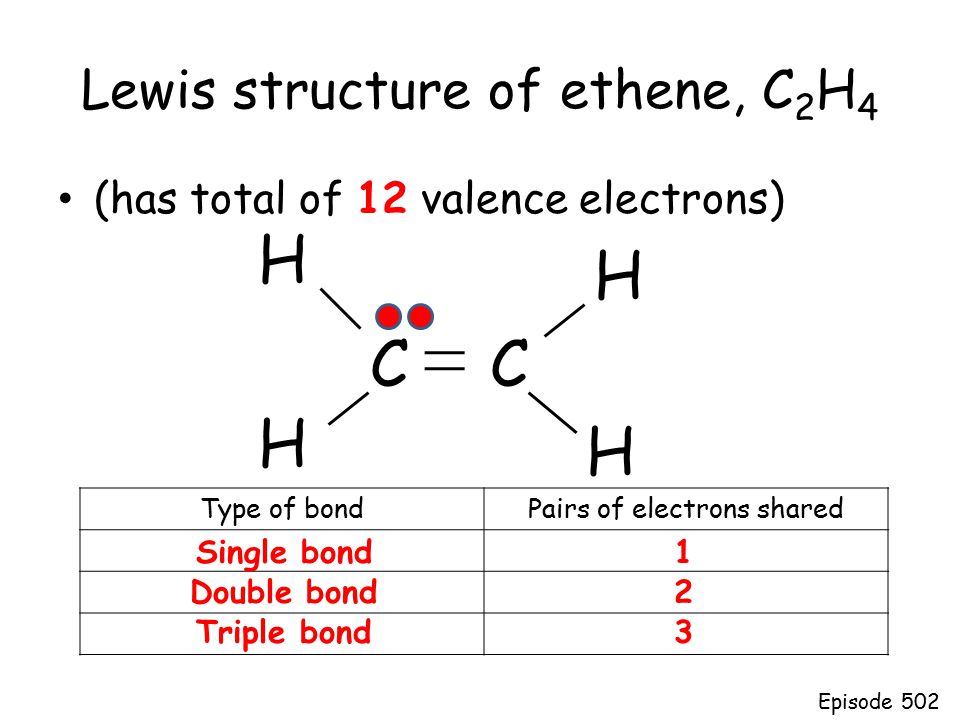
Why is C2H2 a triple bond. We place two valence electrons. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. To attain a stable structure the Carbon atoms will share their remaining three valence electrons by forming a triple bond. Carbon has 4 and hydrogen has 1 making a grand total of 12 e- 42 41.
Hereof how many valence electrons does c2h4 ethene have. For C2H4 you have a total of 12 total valence electrons. It is eight for a single CH4 molecule as four are needed by the carbon atom and one by hydrogen atom each.
For C 2 H 4 you have a total of 12 total valence electrons. Next a search of electrons is required by a single CH4 molecule to reach a stable condition. Find the least electronegative atom and placed it at center.
Sothe total number of C2H4 valence electrons is twelve 8 412These 12 valence electrons have two tasks at the the same timeThey connect all the atoms and satisfy the duet rule of hygrogens and octet rule of two carbon atoms. C2H4 Lewis structure Setup Step-3. Learn more about electron dot structure.
All electrons are shared within bonds in this compound. Carbon has four valence electrons and each Hydrogen atom has one valence electron. Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond.
8C 4H 12 Valence Electrons. With this the dot structure of benzene looks like the structure given in the attachment. The carbon is in group four but sometimes written 14 so it has four valence electrons.
In a double bond two pairs of valence electrons are shared for a. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val. Total valence electrons available for ethene C2H4 lewis structure 42 14 12 valence electrons C2H4 has two carbon and 4 hydrogen atom 2.
It is an oxalate and a dicarboxylic acid dianion. Therefore the total number of valence electrons in Ethylene C 2 H 4. Firstly look for the total number of valence electrons required by a single CH4 molecule which is sixteen.
Ethylene is the simplest molecule that has a double bond. It has a role as a human metabolite and a plant metabolite. Also Know what c2o4 2.
We have 12 available valence electrons. Total number of valence electrons in Ethane Valence electrons of Carbon Valence electrons of Hydrogen 2 4 61 as there are two carbon atoms and six hydrogen atoms we will consider all of them to get the total number of valence electrons 14. A total of six valence electrons is used to form a triple bond between both the.
Oxalate2- is a dicarboxylic acid dianion obtained by deprotonation of both carboxy groups of oxalic acid. Hence there are 14 valence electrons in. Also question is how many valence electrons are in c2o42.
Draw Electron dot structure structural formula of pentene and hexene. To draw the C2H4 Lewis structure follow the below instructions. The carbon atoms and orbitals are.
First of all find out the total number of valence electrons in the C2H4 using the periodic table. It is a conjugate base of an oxalate1-. Ethylene C2H4 involves a double bond between the carbons so each carbon devotes two electrons to its neighboring carbon and two electrons to hydrogens.
Each line in this diagram represents one pair of shared electrons. As we saw from the valence bond model we should find the presence of a σ-bond framework and a. To draw the Lewis structure for C2H4 the total number of valence electrons must be known.
C2h4 Molecular Geometry Shape And Bond Angles Youtube
Ethene C2h4 Lewis Structure Hybridization

The Chemistry Of Organic Compounds Ppt Download

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
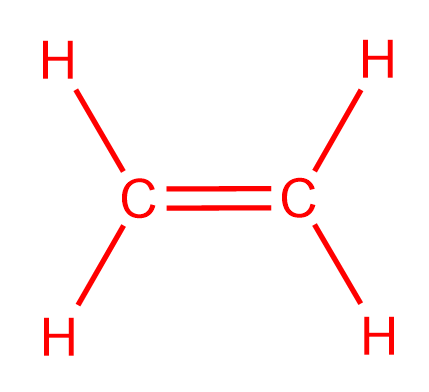
Draw The Lewis Structure For The C2h4 Ske Clutch Prep

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Let S Review Oxygen O 8 1s2 2s2 2p4 Oxygen Has 6 Valence Electrons Ppt Download

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts
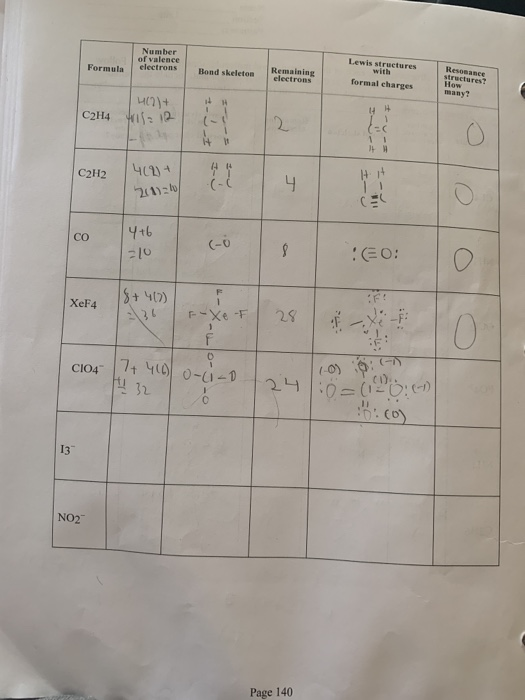
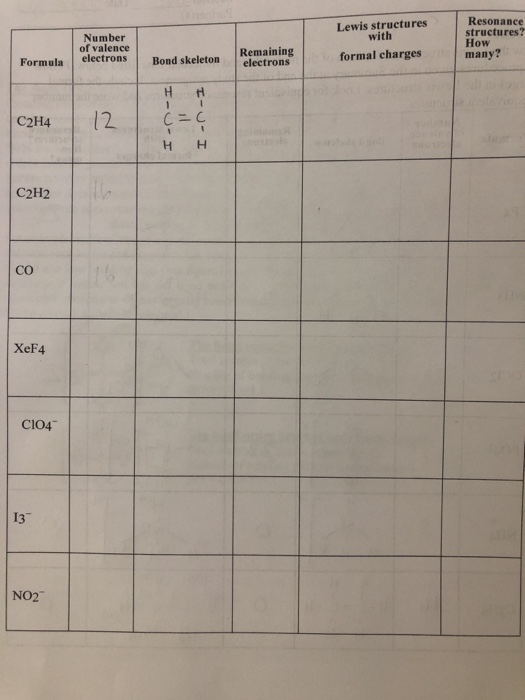
Lewis Structures Number Of Valence Electrons Ressance Chegg Com
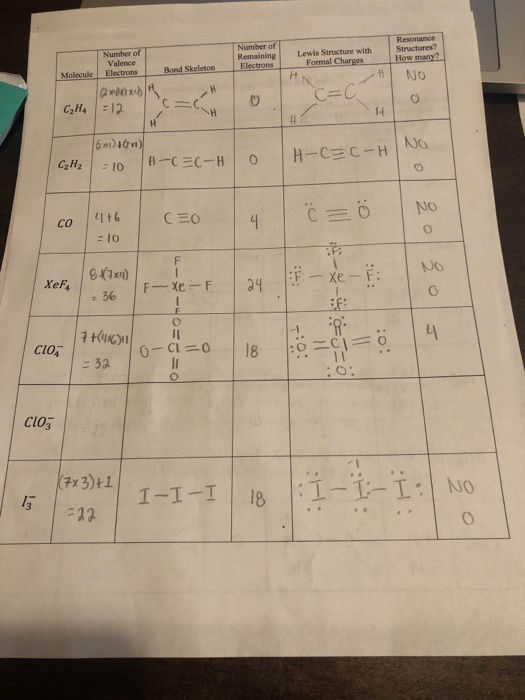
Number Of Valence Electrons Number Of Remaining Chegg Com

Is C2h4 Polar Or Nonpolar Youtube
Ethene C2h4 Lewis Structure Hybridization

Covalent Bonding Write Down The Information On These Slides So That We Can Move Through Them Quickly Tomorrow Ppt Download

Number Of Valence Electrons Lewis Structures With Chegg Com
Https Sacs Instructure Com Courses 13400 Files 607428 Download Wrap 1

Draw The Lewis Structure For The C2h4 Ske Clutch Prep
